Abstract
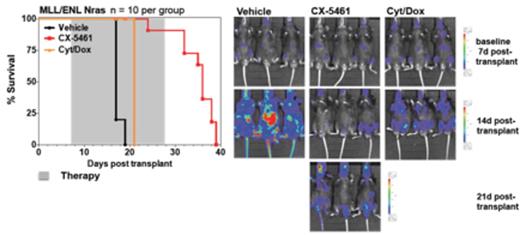
Malignant transformation is commonly associated with dysregulated ribosome biogenesis and for more than 100 years, pathologists have utilized the increase in size and number of nucleoli, the site of ribosome biogenesis, as a marker for aggressive malignancies (Hein et al., Trends in Molecular Medicine, 2013). Recently we demonstrated that hyperactivated RNA Polymerase I (Pol I) transcription, a rate limiting step in ribosome biogenesis, can be specifically targeted by the small molecule inhibitor, CX-5461 (Drygin et al., Cancer Research, 2011; Bywater et al. Cancer Cell, 2012). When evaluated for its anti-proliferative activity against a panel of genetically diverse human cancer cell lines, those derived from p53 wild type hematological malignancies were the most sensitive (Drygin et al., Cancer Research, 2011). Evaluation of whether Pol I transcription inhibition could serve as therapeutic strategy in vivo revealed that CX-5461 administration significantly prolongs the overall survival of Em-Myc lymphoma bearing mice. This survival advantage was associated with rapid activation of the ribosomal protein (Rp)-MDM2-p53 nucleolar stress response (Deisenroth et al., Oncogene, 2010) and subsequent induction of tumour cell-specific p53-dependent apoptosis; importantly the wild type B-cell population was maintained and did not activate p53 pathways (Bywater et al. Cancer Cell, 2012).
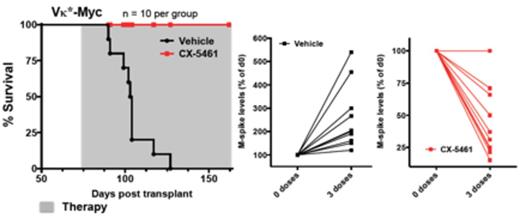
These data demonstrate that hyperactivated Pol I transcription can be successfully targeted through small molecule inhibitors to treat models of human AML and MM that are refractory to standard therapy. Based on these and previously published results (Bywater et al., Cancer Cell, 2012), we have recently initiated a first-in-human, phase I, dose escalation clinical trial of this first-in-class drug, CX-5461 in patients with advanced hematological malignancies. The toxicity, response, pharmacokinetic and co-relative data from this trial will offer valuable insights as to whether inhibition of ribosome synthesis might offer a new non-genotoxic therapeutic approach in the fight against cancers, which are highly refractory to standard therapies.
O'Brien:Senhwa Biosciences, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal