Abstract
The efficacy and safety of LEN in RBC transfusion-dependent pts with International Prognostic Scoring System (IPSS) Low- or Intermediate (Int)-1-risk MDS with del(5q) was assessed in 2 large, multicenter studies (MDS-003 [List A, et al. N Engl J Med. 2006;355:1456-65] and MDS-004 [Fenaux P, et al. Blood. 2011;118:3765-76]). RBC-TI for ≥ 8 weeks and CyR was achieved in 51–67% and 25–73% of pts treated with LEN, respectively. In a multivariate analysis, achievement of RBC-TI ≥ 26 weeks with LEN was associated with a significantly reduced risk of acute myeloid leukemia (AML) progression and death (MDS-004). To further understand the effects of LEN in lower-risk del(5q) MDS pts treated in the MDS-003 and MDS-004 studies, we analyzed the association of CyR with RBC-TI ≥ 26 weeks and AML-free survival.
Of the 286 pts from the MDS-003 and MDS-004 studies who received LEN from study start, 181 were evaluable for both CyR and RBC-TI ≥ 26 weeks, and included in the current analysis (88 pts from MDS-003 and 93 pts from MDS-004). Cytogenetic studies by central review were performed at baseline, and at weeks 24 and 48 (MDS-003); or at week 24 and every 24 weeks thereafter (MDS-004). CyR rates were assessed using International Working Group 2000 criteria and compared with rates of RBC-TI ≥ 26 weeks. CyR and AML-free survival (Kaplan-Meier method) were assessed by baseline cytogenetic complexity [isolated del(5q), del(5q) + 1 additional abnormality, and del(5q) + > 1 additional abnormality]. A univariate Cox proportional hazards model identified factors associated with AML-free survival. CyR and RBC-TI ≥ 26 weeks were included as time varying covariates, and IPSS-R was included as a categorical variable. A best multivariate model was chosen by fitting all possible models, including all covariates with P < 0.1 in the univariate model, and using the Akaike information criterion.
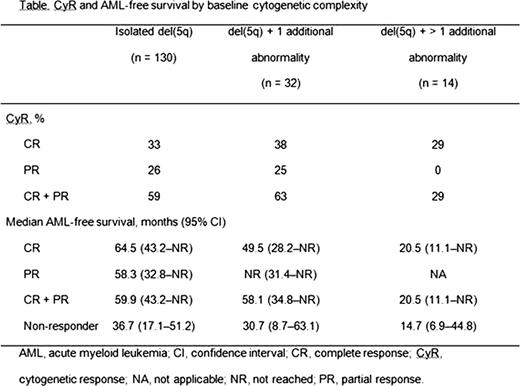
Of the 181 pts, 130 had isolated del(5q), 32 had del(5q) + 1 additional abnormality, 14 had del(5q) + > 1 additional abnormality, and 5 pts had missing cytogenetics at baseline. Median age was 68 years (range 36–89) and 73% of pts were female. Median baseline hemoglobin level was 8.0 g/dL (range 3.6–11.0) and median RBC transfusion burden was 6 units/8 weeks (range 1.0–25.0). A total of 103 pts (56.9%) achieved a CyR (complete + partial response). The likelihood of a CyR was increased in pts who achieved RBC-TI ≥ 26 weeks compared with those who did not: 71% versus 29%, respectively (hazard ratio [HR] 2.3; 95% confidence interval 1.56–3.41). RBC-TI ≥ 26 weeks preceded CyR, with median times to RBC-TI ≥ 26 weeks and CyR of 29 days (range 2–343) and 148 days (range 56–707), respectively. However, this may have been dependent on the timing of bone marrow aspirates. CyR rates were comparable across the isolated del(5q) and del(5q) + 1 additional abnormality groups (59% vs 63%; P = 0.736), but lower in the del(5q) + > 1 additional abnormality group compared to these 2 groups combined (29% vs 60%; P = 0.023) (Table). In pts with isolated del(5q) and del(5q) + 1 additional abnormality, CyR was associated with a 41% (P = 0.019) and 58% (P = 0.056) reduction, respectively, in the risk of AML or death compared with no CyR. In all cytogenetic groups, median AML-free survival was longer in those who achieved CyR compared with non-responders, although it was not statistically significant in pts with del(5q) + > 1 additional abnormality (Table). In a multivariate Cox proportional hazard model, factors associated with AML-free survival were CyR (HR 0.58; P = 0.023), RBC-TI ≥ 26 weeks (HR 0.34; P < 0.0001), lower IPSS-R categorization (P = 0.018), female gender (HR 0.44; P = 0.0004), and younger age (HR 1.03 for each additional year; P = 0.008).
In IPSS-defined Low- or Int-1-risk MDS pts with del(5q), achievement of CyR with LEN was associated with a RBC-TI ≥ 26 weeks response and a reduced risk of AML or death in pts with isolated del(5q) and del(5q) + 1 abnormality. Due to small sample size, further studies are needed to evaluate the effect of LEN in the del(5q) + > 1 additional abnormality group. These data provide evidence for LEN as a disease-modifying agent in RBC transfusion-dependent pts with Low- or Int-1-risk MDS and del(5q).
Sekeres:Celgene: Membership on an entity’s Board of Directors or advisory committees; Amgen: Membership on an entity’s Board of Directors or advisory committees. Swern:Celgene: Employment, Equity Ownership. Giagounidis:Celgene: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees. List:Celgene: Serve on Celgene Data Safety & Monitoring Committee Other. Schlegelberger:Celgene: Consultancy. Fenaux:Celgene: Honoraria. Shiansong Li:Celgene: Employment, Equity Ownership. Sugrue:Celgene: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal