Abstract
Induction and post-remission chemotherapy for acute myeloid leukemia (AML) is an intensive regimen with significant toxicities performed almost exclusively in inpatient or closely monitored outpatient settings. Extreme weight loss is associated with poor outcomes in cancer. We studied whether treated AML patients (pts) lose a substantial amount of weight and whether weight loss affects overall survival (OS).
Adult pts diagnosed with AML at the Cleveland Clinic from 08/08 - 11/12 were included. All pts completed intensive induction chemotherapy with 7+3 (cytarabine and anthracycline – daunorubicin, idarubicin, or mitoxantrone) as first-line therapy. Pts must have achieved complete remission (CR) by first-line induction or day 14 re-induction chemotherapy. Previously treated pts or those with relapsed or refractory disease were excluded. Co-morbidities and AML disease characteristics were assessed, in addition to weight and body-mass index (BMI) at baseline and throughout AML treatment course until disease relapse, stem cell transplantation (SCT), or loss-to-follow-up/death. The primary endpoints were change in BMI relative to completion of post-remission therapy (PRT), OS, and event-free survival (EFS). OS and EFS were measured from completion of PRT to the date of death (OS) and relapse or need for SCT (EFS). To assess impact of weight recovery on OS and EFS separate linear regression models were fit to each pt to determine rate and direction of change during the first 3 months following the completion of PRT. Pts whose fitted regression lines had slopes>0 were considered to have positive weight trajectories; pts with slopes<0, negative trajectories. Group comparisons were made using the Wilcoxon signed-rank test, logrank test, and proportional hazards models.
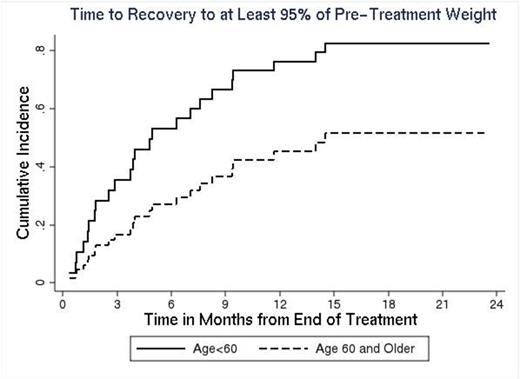
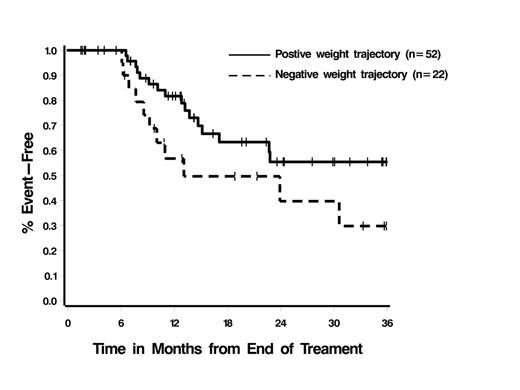
Of 126 AML pts who met the inclusion criteria, 43% (n=54) were male and the median age at treatment start was 54 years (range 18-77). Disease characteristics were: acute promyelocytic leukemia 11% (n=14), other AML 89% (n=112); de novo 75% (n=94), secondary 24% (n=31); cytogenetic risk group - favorable 23% (n=29), intermediate 47% (n=59), unfavorable 21% (n=27), unknown 9% (n=11). Co-morbidities potentially impacting BMI and outcome included diabetes (11%), hypertension (42%), hyperlipidemia (28%), alcohol use (65%), use of steroids (13%) or oral contraceptives (16%). Median weight at the start of therapy was 84.9kg (range 37-159.5) and BMI was 29.6 (range 17.6-57.8). BMI groups included normal (18.5-24.9, 26%), overweight-moderately obese (>25-35, 54%), severely obese (>35, 20%). From the start of treatment, median weight loss was: 2% (range -21-53%, p=0.0001) at day 14; 6% (range -19-22%, p<0.0001) at time of CR determination; 4% (range -23-38%, p<0.0001) at end of PRT. With the exception of diabetes (time of CR, p=0.06) and alcohol use (time of CR and end of PRT, p=0.05 in both cases), none of the clinical or demographic factors examined impacted weight loss. In total, 29% of pts (n=37) had >10% weight loss at the completion of PRT, representing an absolute loss of 5.9-20.6kg; 40% of pts (n=50) had a loss<10%, and 31% of pts (n=39) had no change or weight gain. In the group with >10% loss, median time to recovery of >95% pre-treatment weight was 7 months; 11% of pts (n=4) exceeded their pre-treatment weight by >10%. Only age (p=0.06) was associated with weight recovery time, with younger pts recovering weight faster (Figure 1). 60% of pts (n=73) had disease progression and 36% of pts (n=44) had died; estimated EFS and OS are 10.1 (95% CI 6.6-14.7) and 28.2 months (95% CI 17.1-46.4), respectively. The degree of weight loss following completion of PRT was not associated with EFS (p=0.79) or OS (p=0.82). However, there was some indication that pts with positive weight trajectories had longer EFS than pts with negative trajectories, p=0.07 (Figure 2); OS was not impacted significantly (p=0.26).
AML pts achieving CR with induction therapy experience a median 6% weight loss from start of therapy, but recover by 7 months (median). Those with diabetes or alcohol use lose more weight. Weight changes were not associated with survival; however, there was some suggestion that pts who began to gain weight once PRT was completed had longer EFS than pts whose weight continued to decline. These data can be used to frame pt expectations about weight fluctuations going through AML treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal