Abstract
Molecular and cytogenetic abnormalities characterize the biologic heterogeneity of B-ALL and provide a strong and independent prognostic factor for treatment outcome. Emerging data highlight the role of tyrosine kinase inhibition (TKI) in those with Philadelphia chromosome positive (Ph+) B-ALL. A new high risk subset, so-called Ph-like ALL, has been recognized, comprising 10-15% of pediatric B-ALL. These cases show similar alterations in IKZF1, and are likely driven by alternative kinase rearrangements, including CRLF2, JAK2, ABL1, and others. Increased STAT5 phosphorylation may broadly classify such patients, and, also suggest a benefit with JAK2 inhibitors or TKI’s. Finally, c-MYC has been recognized as a high risk feature for both B- and T-ALL, though it is unclear whether the risk is similar among Ph+ and Ph- patients in the era of TKI-based therapy.
A retrospective analysis was performed of a series of patients at Moffitt Cancer Center with B-ALL diagnosed between January 2003 and June 2012. Of them, paraffin embedded blocks from patients who had the first diagnostic bone marrow biopsy performed at MCC with a good biopsy quality (> 1.0 cm in length and tumor load over 70% of marrow cellularity) were selected for performing immunohistochemical (IHC) study. Ten normal bone marrows were also included as antibody base-line control. IHC stains were performed according to institutional standard protocol and modified based on the individually purchased antibody. Expression of pSTAT5, c-MYC, and IKZF1 on lymphoblasts was assessed semiquantitatively based on the expression score calculated with staining strength and multiplied by percentage positivity on tumor cells. Clinical outcomes were correlated to the IHC scores. Hazard ratios were generated using standard cox regression analysis. Patient survival was analyzed with Kaplan-Meier method from the date of diagnosis until death from any cause.
29 B-ALL patients, including 15 Ph+ and 14 Ph-, with median age 58years (19 - 72), median white blood cells (WBC) 10.1 k/uL (1.8-200), and male to female ratio of 2:1, were included in the study. 86% received HyperCVAD induction, and 93% of Ph+ patients received TKI therapy, most commonly with Imatinib (86%), and 32% received an allogeneic transplant in first remission. No significant difference in age, WBC, or receipt of allogeneic transplant, time to progression (TTP), or overall survival OS was apparent when comparing Ph+ to Ph- patients.
The IHC staining results showed nuclear expression of pSTAT5 to be more frequently present in Ph+ B-ALL than in Ph- B-ALL (73% vs 33%, p = 0.0574), however, no differences in staining were observed with IKZF1 (80% vs 92%, p=0.61) or c-MYC (33% vs 50%, p=0.41).
Overexpression of c-MYC was associated with decrease in OS (HR 4.1, 95%CI 1.2-13.9, p=0.02)(Figure 1), with a trend towards decreased TTP (HR 4.3, 95% CI 0.8-22.2, p=0.08). Neither pSTAT5 nor IKZF1 were predictive of OS or TTP when analyzing the entire cohort.
Further analysis of the Ph+ group demonstrated a trend for impaired OS among c-MYC positive patients (HR 4.3, 95%CI 0.8-22.2, p=.08). There was no significant impact of c-MYC on TTP, and no impact of pSTAT5 or IKZF1 expression in regards to TTP or OS. 23% Ph+ patients and 7% Ph- patients showed loss of IKZF1 expression (p=0.61).
Further analysis of the Ph- group demonstrated a trend towards impaired TTP among those with high expression of pSTAT5 (HR 9.4, 95%CI 0.8-105.8, p=0.07). There was no impact of pSTAT5 on OS, and no impact of IKZF1 or c-MYC expression in regards to TTP or OS.
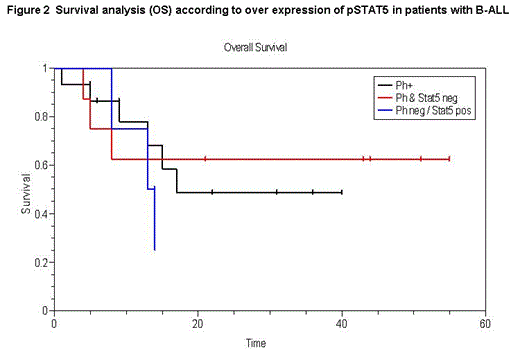
Ph+ B-ALL patients more commonly expressed pSTAT5, but this did not influence outcome, perhaps due to TKI exposure. A subset of Ph- patients with high pSTAT5 had a trend towards lower OS in comparison to Ph- and pSTAT5 negative patients (figure 2). This may be a novel group for the study of TKI or JAK2 inhibitors. Expression of c-MYC was associated with impaired OS and a trend towards lower TTP, an effect which was most pronounced in the Ph+ group.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal