Abstract
Acute myeloid leukemia (AML) is a heterogeneous disease marked by a highly variable clinical course and response to therapy. microRNA's (miR) have entered the spotlight for being involved in pathogenesis of numerous diseases, including hematopoietic malignancies. miR-125a-5p previously was identified to be decreased in AML, however its functional role leading to the pathogenesis of AML is unknown leading us to dissect its functional role in AML within these studies.
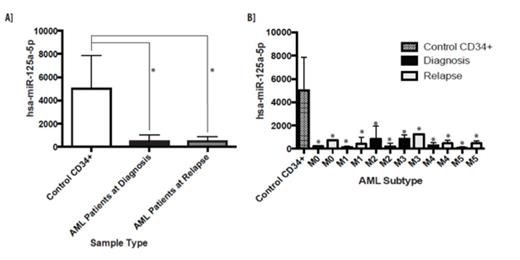
Small RNA sequencing illustrated that miR-125a is decreased in many AML FAB subtypes at time of diagnosis and relapse (p<0.05) beyond the previously reported cytogenetically normal AML (Figure 1A&B). miR-125a expression in comparison to clinical aspects (167 AML patients) was conducted using data from the National Cancer Institute the Cancer Genome Atlas (TCGA) Data Portal to support small RNA sequencing findings. The majority of patients had low miR-125a expression (1-250 RPM), which is significantly decreased if compared to the small RNA sequencing results for healthy CD34+ cells (∼5000RPM). Interestingly, there is no difference between gender and miR-125a expression. Though not significant, there was a trend of low miR-125a expression towards low survival rate. To dissect the pathways affected when miR-125a expression is restored in AML, acute promyelocytic leukemia (APL) NB4 cells containing t (15; 17), which had the most significantly decreased expression when AML lines were screened, were utilized for studies. Previously we reported that miR-125a was epigenetically silenced in AML and caused altered cell proliferation, cell cycle progression, and apoptosis, however through RNA expression profiling we now know the potential players altered leading to these altered biological pathways. Among the significant decreased genes were FLT1, MMP-9, IL-32Rα and HIP-1 while several increased genes were of interest such as cathepsin-G, EPX, and SPARC. Though all of these were interesting due to their previous implications within cancer or AML, they are not predicted targets of miR-125a. Therefore, we focused our analysis on identifying a potential target of miR-125a within AML.
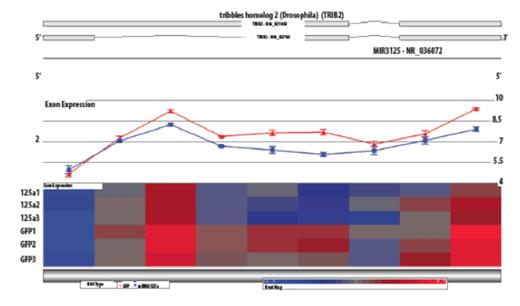
From profiling results, Trib2 was significantly decreased (p=0.0003, Figure 2) when miR-125a expression was restored in NB4 cells and is a predicted target. RT-qPCR and 3'UTR luciferase confirmed that Trib2 was a target of miR-125a. Trib2 has been implicated in AML in several contexts including inhibition of C/EBPα causing decreased cell differentiation and its ability to interact with HoxA9 to aid in the progression of AML. Although Trib2 has been implicated in cancer, inhibitors are not developed currently but a necessity has been demonstrated. Therefore, we focused our studies on identifying a pathway with known inhibitors. Several reports demonstrate enhanced ErbB2 expression when miR-125a is decreased leading us to test Mubritinib, which selectively inhibits ErbB2 phosphorylation. Previously we demonstrated the profound affect on inhibition of cell cycle progression and altered cell proliferation, differentiation, and apoptosis in NB4 cells. Most strikingly was the lack of affect of the inhibitor on HL60 cells, which do not have decreased miR-125a expression like NB4 cells suggesting that inhibition of the ErbB pathway would be specific for low miR-125a AML. Excitingly, ANOVA pathway analysis revealed the ErbB pathway was significantly altered, such as decreased ErbB receptors (ErbB1 and ErbB3) and downstream effectors (PI3K, AKT, and Stat5). After establishing the role of the ErbB pathway in NB4 cells we tested the effect of Mubritinib on retinoic acid resistant NB4 cells (NB4-LR1), which is indicating that Mubritinib could potentially be utilized for a new therapeutic treatment in NB4-LR1 cells in addition to NB4 cells.
Decreased miR-125a within AML may give leukemic blasts an advantage in multiple AML subtypes. Through RNA expression profiling, Trib2 was identified as a target of miR-125a within AML. By utilizing pathway analysis in we have identified a potential new therapeutic, Mubritinib, for miR-125a low AML, which is now being tested in vivo. From our strong preliminary work ErbB inhibitors currently being utilized for treating ErbB overexpressing epithelial cancers could be tested in hematopoietic malignancies in addition to identifying a potential role of Trib2 in miR-125a low AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal