Abstract
Phosphorylation of signal transducer and activator of transcription 3 (STAT3) is essential for cell survival, proliferation and differentiation. STAT3 phosphorylation results from signaling by cytokines and growth factors, and constitutive STAT3 activity is characteristic of a number of human malignancies, including Cutaneous T Cell Lymphoma (CTCL). Furthermore, we now know that STAT3 is also required for the initiation and maintenance of the Th17 differentiation program. Th17 cells are a subset of CD4 T helper cells that have been implicated in chronic inflammatory conditions like rheumatoid arthritis and psoriasis. Mycosis fungoides (MF) and the leukemic variant of this disease, Sezary syndrome (SS), are the most frequently encountered forms of CTCL and in both of these diseases, the cell of origin – as far as the type of Teffector cell involved, has not been defined. Recent results from our laboratory and that of our colleagues have lead us to believe that Th17 cells may either be the cells of origin in CTCL or may act as critical mediators of chronic inflammation that creates a favorable environment for tumor growth in the context of this malignancy.
In an effort to elucidate the role of STAT3 as a transforming factor in T cell malignancies, we generated a mouse model wherein T cell specific expression of a hyper-active STAT3 mutant protein (STAT3C) leads to the development of a lymphoproliferative disease that is highly reminiscent of CTCL. We are now taking advantage of this unique mouse model, patient biospecimens and carefully characterized CTCL cell lines to dissect the role of STAT3 signaling cascade in the malignant transformation and maintenance of CTCL.
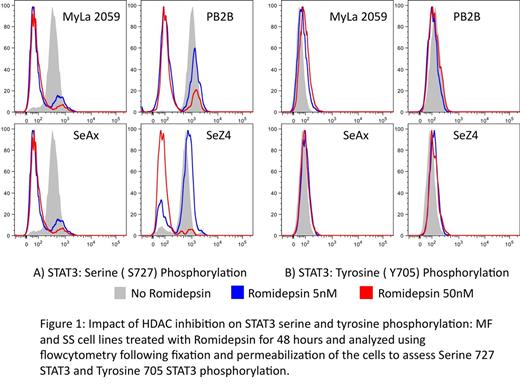
The role of serine phosphorylation in the context of STAT3 signaling is hotly debated and we are now attempting to characterize the role of Serine STAT3 phosphorylation in the context of CTCL. We are also hoping to recapitulate these observations in patients' biospecimens collected before and after treatment with HDAC inhibitors. We will also study the role of serine phosphorylation in STAT3 activity in carcinogenesis using our mouse model with phenotypic and pathological characteristics similar to CTCL. We hope that these studies will advance our knowledge about the role that Stat 3 signaling plays in MF/SS malignant transformation and cancer progression and help us develop target specific treatment options for the clinical practice.
Hymes:Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal