Abstract
Stem cells play key roles in early normal development (e.g. embryonic stem cells (ESCs)), maintenance of adult organs (e.g. hematopoietic stem cells (HSCs)) and in some cancers (e.g. leukemia stem cells). To what degree these different types of stem cells rely upon shared versus distinct transcriptional programs remains controversial. Sall4 is a zinc finger transcription factor that exists in two distinct splice isoforms, Sall4a (long) and Sall4b (short). Sall4 has been implicated in embryonic, hematopoietic and malignant stem cell transcriptional regulation. Additionally, Sall4 has been proposed as a potential means of ex-vivo hematopoietic stem cell expansion prior to transplantation. Sall4 isoform-specific differences have been described in ESCs, with Sall4b shown to be critical for maintaining ESC “stemness”. Here we investigate the role of Sall4 isoforms in pediatric acute myeloid leukemia (AML) and murine hematopoiesis to unravel shared versus unique transcriptional programs across different stem cell types.
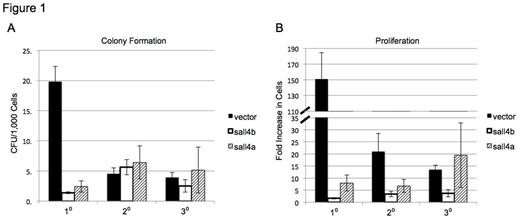
Lin- bone marrow expressing Sall4a, Sall4b or empty vector was cultured in methylcellulose; plates were flushed and replated out to three generations. Colony forming units were assessed (A) and viable cells were counted (B) after 7-10 days in culture.
Lin- bone marrow expressing Sall4a, Sall4b or empty vector was cultured in methylcellulose; plates were flushed and replated out to three generations. Colony forming units were assessed (A) and viable cells were counted (B) after 7-10 days in culture.
In conclusion, our data shows that Sall4b is expressed in murine hematopoietic stem cells and progenitors, suggesting that Sall4b but not Sall4a influences a hematopoietic cell fate. Additionally, Sall4 expression is variable in AML specimens, implicating a potential pathogenic role in some leukemias, while others are Sall4-independent. Lastly, Sall4 overexpression is associated with decreased expression of the critical hematopoietic gene Bmi1. Together this data suggests that hematopoiesis is dependent upon appropriately regulated Sall4 expression with alterations leading to impaired proliferation and self-renewal. These effects on hematopoiesis appear to be mediated at least in part through a dose-dependent effect on Bmi1 expression. Future studies will evaluate other genes targeted by Sall4 in hematopoiesis and leukemia to define Sall4-dependent gene signatures in normal versus malignant hematopoiesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal