Abstract
At the end of VTE treatment, increasing D-Dimer levels after discontinuation of Vitamin-K antagonists (VKA) have been shown to indicate coagulation activation and increased risk of VTE recurrence. However, it is unknown if changes of coagulation activation parameters will be similar after discontinuation of direct oral anticoagulants (DOAC) such as apixaban, rivaroxaban and dabigatran. Furthermore, the clinical impact of these changes is still unclear.
To quantify changes of coagulation activation parameters at the end of VTE treatment with VKA or DOAC and to evaluate their positive predictive value for VTE recurrence at 12 months.
Blood samples for coagulation tests were collected from consenting patients with proximal VTE who discontinued anticoagulation treatment at the end of apixaban, dabigatran or rivaroxaban phase-III VTE treatment trials. Furthermore, similar samples were obtained from VKA patients at the end of treatment.
From all patients, samples for D-dimer (DD), prothrombin fragments (F1+2) and thrombin-antithrombin complexes (TAT) measurements were collected at the end of treatment and 4 weeks later. Samples were analysed by blinded lab personnel and statistically evaluated for differences between VKA and DOAC regarding changes between both samples as well as absolute values at 4 weeks. Finally, all patients underwent 12 months follow-up by phone calls to establish rates of recurrent VTE or death from any cause.
Blood samples were obtained from patients discontinuing apixaban (A; n=37), dabigatran (D; n=17), rivaroxaban (R; n=9) and VKA (n=184), respectively.
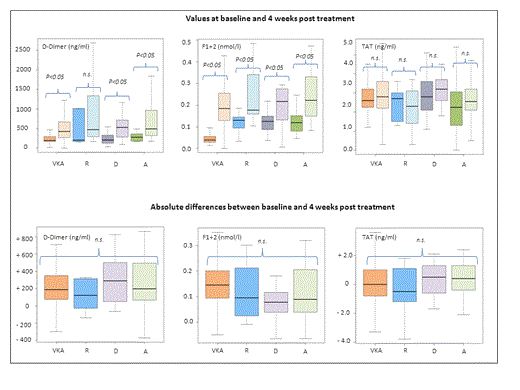
Absolute values and relative changes of DD, F1+2 and TAT at baseline and 4 weeks were not significantly different between VKA or the DOAC cohorts. Irrespective of the anticoagulant treatment, DD and F1+2 but not TAT demonstrated a significant increase between baseline and week 4 (figure 1).
DD, F1+2 and TAT values at baseline and 4 weeks after discontinuation of VKA, rivaroxaban (R), dabigatran (D) and apixaban (A) VTE treatment. Upper row: increase between baseline and 4 weeks (all significant); lower row: comparison of absolute changes between treatment groups (all not significant)
DD, F1+2 and TAT values at baseline and 4 weeks after discontinuation of VKA, rivaroxaban (R), dabigatran (D) and apixaban (A) VTE treatment. Upper row: increase between baseline and 4 weeks (all significant); lower row: comparison of absolute changes between treatment groups (all not significant)
At 12 months, 18 patients (7.3%) had recurrent VTE and 2 patients (0.8%) were dead. Regarding clinical outcomes at 12 months, the negative predictive values (NPV) of DD, F1+2 and TAT were highest for patients after VKA treatment (at least 0.93) and systematically lower for DOAC patients (ranging between 0.86 and 0.91).
In contrast, positive predictive values (PPV) of DD, F1+2 and TAT were systematically higher in DOAC patients (0.19 to 0.43) compared to VKA patients (0.03-0.16) with highest values for TAT-complexes > 200% baseline (PPV VKA 0.14; PPV DOAC 0.43), which was also seen in logistic regression analysis with a significant risk increase for VTE/death (Odds ratio for TAT > 200% baseline 5.0; p=0.006). None of the other parameters showed a correlation to the risk of recurrent VTE or death.
Changes of DD, F1+2 and TAT values post treatment are not different between patients discontinuing VTE treatment with VKA, apixaban, dabigatran or rivaroxaban. NPV of DD, F1+2 and TAT for recurrent VTE/death are higher in VKA than DOAC patients, while PPV are higher in DOAC patients.
At 4 weeks, a TAT increase over 200% of baseline value was found to be associated with a 5-fold increase of recurrent VTE or death with a PPV of 0.14 for VKA patients and of 0.43 for DOAC patients.
negative (NPV) and positive (PPV) predictive values of absolute values and relative changes of D-dimer, prothrombin fragments (F1+2) and thrombin-antithrombin complexes (TAT) at end of anticoagulant treatment (baseline) and 4 weeks after.
| Parameter . | All n=247 NPV/PPV . | VKA n=184 NPV/PPV . | DOAC n=63 NPV/PPV . |
|---|---|---|---|
| Baseline D-dimer< / > 501 ng/ml (ULN) | 0.93/0.15 | 0.94/0.08 | 0.89/0.37 |
| 4week D-dimer< / > 501 ng/ml (ULN) | 0.94/0.11 | 0.95/0.07 | 0.91/0.19 |
| Δ D-dimer< / > 200% baseline value | 0.93/0.09 | 0.93/0.05 | 0.91/0.19 |
| Baseline F1+F2< / > 0.229 nmol/l (ULN) | 0.92/0.2 | 0.94/n.a. | 0.86/0.25 |
| 4week F1+F2< / > 0.229 nmol/l (ULN) | 0.92/0.08 | 0.93/0.03 | 0.89/0.19 |
| Δ F1+F2< / > 200% baseline value | 0.91/0.08 | 0.93/0.06 | 0.90/0.22 |
| Baseline TAT< / > 4.1 ng/ml (ULN) | 0.93/0.14 | 0.94/0.05 | 0.89/0.33 |
| 4week TAT< / > 4.1 ng/ml (ULN) | 0.93/0.18 | 0.96/0.16 | 0.87/0.25 |
| Δ TAT< / >200% baseline value | 0.93/0.21 | 0.95/0.14 | 0.88/0.43 |
| Parameter . | All n=247 NPV/PPV . | VKA n=184 NPV/PPV . | DOAC n=63 NPV/PPV . |
|---|---|---|---|
| Baseline D-dimer< / > 501 ng/ml (ULN) | 0.93/0.15 | 0.94/0.08 | 0.89/0.37 |
| 4week D-dimer< / > 501 ng/ml (ULN) | 0.94/0.11 | 0.95/0.07 | 0.91/0.19 |
| Δ D-dimer< / > 200% baseline value | 0.93/0.09 | 0.93/0.05 | 0.91/0.19 |
| Baseline F1+F2< / > 0.229 nmol/l (ULN) | 0.92/0.2 | 0.94/n.a. | 0.86/0.25 |
| 4week F1+F2< / > 0.229 nmol/l (ULN) | 0.92/0.08 | 0.93/0.03 | 0.89/0.19 |
| Δ F1+F2< / > 200% baseline value | 0.91/0.08 | 0.93/0.06 | 0.90/0.22 |
| Baseline TAT< / > 4.1 ng/ml (ULN) | 0.93/0.14 | 0.94/0.05 | 0.89/0.33 |
| 4week TAT< / > 4.1 ng/ml (ULN) | 0.93/0.18 | 0.96/0.16 | 0.87/0.25 |
| Δ TAT< / >200% baseline value | 0.93/0.21 | 0.95/0.14 | 0.88/0.43 |
n.a.= not applicable (no events)
Werth:Bayer Healthcare: Honoraria. Beyer-Westendorf:Bayer Healthcare: Research Funding, Speakers Bureau; Boehringer Ingelheim: Research Funding, Speakers Bureau; Pfizer: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal