Abstract
We have recently reported that event-free survival at 24 months from diagnosis (EFS24) is a robust endpoint for assessment of disease related outcome for newly diagnosed patients with diffuse large B-cell lymphoma (DLBCL) treated with anthracycline-based immunochemotherapy (IC). Patients who achieve EFS24 following IC have a subsequent overall survival that is equivalent to the age and sex matched general population. The IPI was developed in the pre-rituximab era using OS/EFS endpoints. While the IPI is associated with outcome in the IC era, the original survival estimates for the IPI risk groups are outdated. The IPI is also a discrete risk classifier, with only 6 possible groups. Here we remodel the IPI using EFS24 as the endpoint in a large international cohort of patients treated with IC. This model provides an individual level 0-100% risk of failing to achieve EFS24 for each patient and can be used for patient prognosis, treatment stratification, and risk assessment.
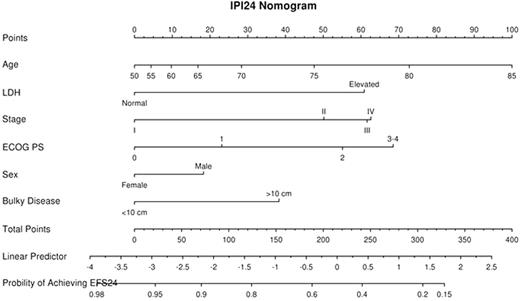
Newly diagnosed DLBCL patients treated with immunochemotherapy from the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource, NCCTG N0489 clinical trial, GELA LNH2003B program, and a Lyon, France based hospital registry were included in the original EFS24 study. Patients with a relapse, progression, unplanned re-treatment, or death due to any cause within 730 days of diagnosis were considered a failure for the EFS24 endpoint. Patients alive and progression/relapse free at 730 days from diagnosis with no subsequent treatment following initial IC were considered a success for the EFS24 endpoint. Variables selected for potential model inclusion were the IPI components (age, stage, ECOG PS, number of extranodal sites, and LDH), as well as standard clinical variables typically reported in studies of DLBCL: presence of B-symptoms, bone marrow involvement, bulky disease (>10 cm), and sex. The IPI24, a prognostic model for EFS24, was developed using logistic regression models. Model building began by fitting the classic IPI score (0-5), then allowing individual IPI variables to carry different model weights and number of categories, followed by dropping insignificant variables, finally adding any additional significant variables. Model performance was assessed using bias corrected c-statistics, AIC, and R2 values. Model selection was done using the complete case set with multiple imputation on the full dataset used to refine parameter estimates for the final model.
1596 total patients were available for modeling. The median age was 62 (range 18-93) and 53% were male; 33% were IPI 0-1, 25% were IPI 2, 25% were IPI 3, and 17% were IPI of 4-5. The failure rate for achieving EFS24 was 29%. All variables but sex (p=0.33) were univariately associated with EFS24 (p<6.4x10-5). The original IPI had good prognostic ability for EFS24 (c=0.696, p=2.2x10-32). Allowing multiple categories for ECOG PS, stage, and age (continuous) improved model fit and prognostic ability (c=0.727). The number of extranodal sites was not significant when IPI variables were modeled separately and was removed from the model. Sex and bulky disease were additional significant variables when added to the model. Thus, the final IPI24 model contained the following risk predictors: male sex, ECOG PS (0 vs. 1 vs 2 vs 3-4), stage (I vs. II, vs. III vs. IV), LDH (elevated vs. normal range), bulky disease > 10cm, and age (continuous). This model resulted in improved fit and prognostic ability (c=0.749) over the standard IPI (c=0.696, bootstrap p<0.001). Model validation was performed on an additional 158 patients from the SPORE MER and showed good concordance (c=0.697). Additional validation is planned in a larger set of independent patients.
The IPI24, generated using standard clinical variables, yields an individual risk prediction for failing to achieve EFS24 following IC for treatment of DLBCL. The IPI24 has superior prognostic ability to the standard IPI and can be implemented in the clinic using a nomogram or as a simple electronic application.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal