Abstract
Recombinant thrombomodulin (rTM), a new type of natural anticoagulant, has been available in Japan since 2008 for the treatment of disseminated intravascular coagulation (DIC). Since limited information is available on its efficacy in children, we compared the efficacy of rTM to that of conventional treatment.
Of 41 children with DIC, 21 were treated with rTM as a first-line therapy (rTM group) and 20 weere treated with conventional treatment (control group). The day on which anti-DIC treatment was first given was defined as day 1. We used the DIC criteria of the Japan Welfare and Health Ministry. We evaluated the overall survival at day 28 after anti-DIC treatment, the cumulative incidence of DIC resolution, and the total fresh frozen plasma (FFP) and platelet transfusion volumes during the course of DIC.
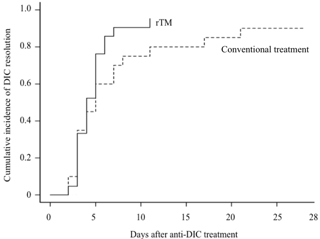
Clinical outcomes are summarized in the Table. Both groups showed comparable overall survival (95.2% in the rTM group and 95.0% in the control group). In addition, the cumulative incidence of DIC resolution in the rTM group (90.5%) was not different from that in the control group (70.0%) (p=0.210) (Figure). However, the median FFP and platelet transfusion volumes in the rTM group (0 U/kg and 0.28 U/kg) were lower those in the control group (0.14 U/kg and 0.76 U/kg) (p=0.041 and 0.063, respectively).
Comparison of the clinical outcomes between rTM group and the control group
| . | rTM group (n=21) . | Contro group (n=20) . | p . |
|---|---|---|---|
| Survival at day 28 after anti-DIC treatment | 95.2 % (95% CI; 86.6-100) | 95.0 % (95% CI; 85.9-100) | 0.986 |
| Bleeding events during DIC | 0 | 1 | 0.98 |
| Cumulative incidence of DIC resolution at day 7 | 90.5 % (95% CI; 75.9-100 ) | 70.0 % (95% CI; 48.9-91.1) | 0.21 |
| Median time until DIC resolution (days) | 4 | 5 | |
| Treatment failure | |||
| Death within 28 days after diagnosis of DIC | 1 | 1 | |
| Sustained DIC status at day 7 but not dead at day 28 | 1 | 5 | |
| Median platelet infusion volume during DIC (U/kg) | 0.28 (range; 0-1.67) | 0.76 (range; 0-43.3) | 0.063 |
| Median FFP infusion volume during DIC (U/kg) | 0 (range; 0-0.44) | 0.14 (range; 0-10.27) | 0.041 |
| . | rTM group (n=21) . | Contro group (n=20) . | p . |
|---|---|---|---|
| Survival at day 28 after anti-DIC treatment | 95.2 % (95% CI; 86.6-100) | 95.0 % (95% CI; 85.9-100) | 0.986 |
| Bleeding events during DIC | 0 | 1 | 0.98 |
| Cumulative incidence of DIC resolution at day 7 | 90.5 % (95% CI; 75.9-100 ) | 70.0 % (95% CI; 48.9-91.1) | 0.21 |
| Median time until DIC resolution (days) | 4 | 5 | |
| Treatment failure | |||
| Death within 28 days after diagnosis of DIC | 1 | 1 | |
| Sustained DIC status at day 7 but not dead at day 28 | 1 | 5 | |
| Median platelet infusion volume during DIC (U/kg) | 0.28 (range; 0-1.67) | 0.76 (range; 0-43.3) | 0.063 |
| Median FFP infusion volume during DIC (U/kg) | 0 (range; 0-0.44) | 0.14 (range; 0-10.27) | 0.041 |
rTM; recombinant thrombomodulin, DIC; disseminated intravascular coagulation, FFP: fresh frozen plasma
Cumulative incidence of DIC resolution
The use of rTM in children with DIC should reduce the social and ethical problems as well as the biological risks associated with human-derived blood products. Although the limited advantages of rTM were shown, the present study was limited by the small and heterogeneous patient population. Therefore, larger prospective studies are warranted to fully evaluate the efficacy of rTM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal