Abstract
Acquired hemophilia A (AHA) is a rare severe disorder caused by autoantibodies against FVIII resulting in significant morbidity and mortality up to 20 % in some studies. The two pillars of treatment include acute bleeding control, and immunosuppressive therapy which aims to stop the underlying autoimmune phenomenon. Although critical for long-term disease-free survival, there is currently no consensus for the best immunosuppressive regimen. Most authors use steroids first line, followed by cyclophosphamide and/or rituximab in steroid failures. However, upfront low-dose combined regimens offer the theoretical advantage of reduced steroid-exposure and toxicity as well as increased effectiveness. In the current retrospective analysis, we aim to review our institutional experience with such a regimen.
We identified 16 (7 males and 9 females) AHA patients newly diagnosed between October 2009 and June 2013; all treated with an identical regimen on an institutional protocol. Patient age varied between 53-86 (median 76,4) years. Mean initial inhibitor titer was 30.5 Bethesda units (BU; range 2 - >500). In 4 out of 16 patients (26,7 %) an underlying disease was identified, the remaining 12 cases were considered idiopathic. Low-dose combined immunosuppression consisted of the following regimen: 1000 mg cyclophosphamide on day 1 and 22, 40 mg dexamethasone on day 1, 8, 15 and 22, and 100 mg rituximab on day 1, 8, 15 and 22 (the regimen was termed CyDRi). Simultaneous use of bypassing agents was left to the discretion of the treating physician (8/16 patients, 50 %). All patients received at least 1 cycle of CyDRi. If complete remission (CR, defined as cessation of bleeding and an undetectable FVIII inhibitor in the Bethesda assay) was not achieved, the CyDRi therapy was repeated every 6 weeks until remission. Laboratory follow-up included CBC, routine chemistry and APTT (with a mixing study when prolonged), as well as FVIII levels and the Bethesda assay. Clinical monitoring varied according to the localization of bleeding.
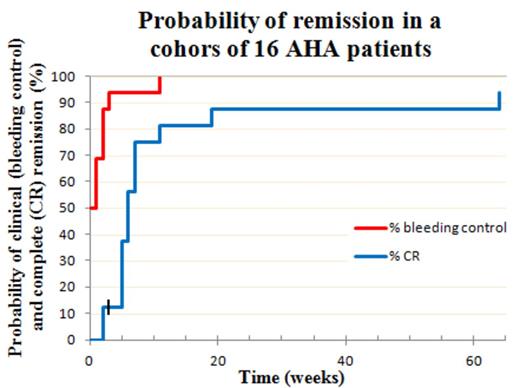
50 % of patients presented with severe active bleeding symptoms, and they all received bypassing agents simultaneously with the CyDRi regimen. The remaining 8 patients had a recent history of bleeding but were not actively bleeding at the time of admission, and were given CyDRi without concurrent bypassing agents – none of them had a subsequent episode of bleeding (time to bleeding control = 0; Figure) Bleeding control was also rapidly achieved in the other patients with >90 % of patients free of bleeding within 3 weeks (Figure). CR was achieved in 15/16 patients (93,75 %). The time to CR was short with more than 75% of patients in CR by week 7 (Figure). Toxicity and side effects were infinitesimal compared to commonly used prolonged steroid therapies: One patient acquired pneumonia three weeks after discharge from the hospital, and two patients developed Clostridium difficile colitis during hospitalization. All three patients responded to antibiotic treatment. 8 of 16 patients (50 %) required more than one cycle of CyDRi to achieve a durable CR, either due to slow response or a laboratory relapse (6 and 2 patients, respectively). Two of the 8 patients required a third cycle of CyDRi (one for a very slow response, the other for a second relapse). CyDRi was equally effective in treating relapses. One patient died from a cause deemed unrelated to AHA (elderly patient with a femoral neck fracture). Her inhibitor titer was falling rapidly, but she died before achieving CR. Follow-up of our cohort is median 21 (range 2-45) months.
The CyDRi low-dose combined immunosuppression produced higher complete remission rates with a remarkably rapid cessation of bleeding and a significantly improved side-effect profile and mortality rate in this retrospective AHA cohort, compared to regimens previously described in the literature. A prospective randomized comparison seems warranted to confirm these results.
Off Label Use: Cyclophosphamide, dexamethasone and rituximab are all off label use for the treatment of acquired hemophilia A.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal