Abstract
TRAs increase platelet counts by stimulating the TPO-receptor. A known effect of TRA treatment is increased bone marrow fibrosis (MF). This study explored extent of MF, its clinical relevance, and incidence of phenotypic or karyotypic abnormalities in TRA-treated ITP patients.
This single-center study was carried out at the Platelet Disorders Center of Weill Cornell Medical College (WCMC), NY, USA. Eligibility criteria were: diagnosis of ITP; treatment with a TRA (romiplostim, eltrombopag, AKR 501 (Eisai) or Shionogi agent), ≥ 1 bone marrow biopsy (BMB) performed during TRA treatment. BMBs were performed every 1–2 years as standard f/u procedure for our ITP patients on TRA. MF grade was assessed from MF-0 to MF-3 according to the European Consensus Grading System in 141 BMBs acquired prior to (n=15), during (n=117) and after (n=9) TRA-treatment from 66 patients. Fifty disease-free staging BMBs served as controls.
BMBs were separately reviewed by 3 pathologists to assess the grade of MF and then reviewed concurrently as needed to reach consensus. The study was approved by the IRB of WCMC; informed written consent was obtained from patients.
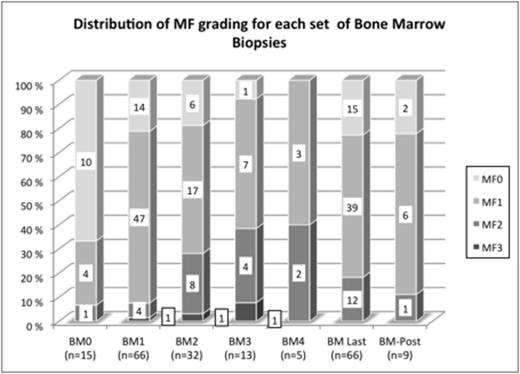
Median (Q1-Q3) age at the time of 1st BMB was 38 years (18-63); 34 males 32 females. 32 patients had > 2 on-treatment BMBs. The distribution of MF-grades is shown in the figure.
The proportion of MF-0 decreased from 67% in pretreatment biopsies (BM0) to 21% in the first set of BMBs (BM1); in the 15 patients with pre- and on-treatment BMBs there was a significantly higher number of MF-0 in BM0 as compared to BM1 (10/15 vs. 3/15;p=0.016) suggesting that TRAs induce fibrosis in treated patients. In patients with multiple on-treatment BMBs (n=32), first on-treatment BMB was graded as MF-1 in 24.
In the last set of biopsies (BM-Last) 8 had progressed to MF-2/3, 12 remained MF-1, and 4 became MF-0 illustrating the unpredictability of the future course of MF from the first on-treatment marrow. Nonetheless, a higher number of MF-2/3 BMB was found in BM-Last as compared to BM1 [10 (31%) vs. 3 (9%) of 32; p=0.039].
In 5 patients with MF-2/3 BMB, TRA were discontinued: on f/u 2 had less fibrosis, 1 remained the same, and 2 are awaiting f/u BMB.
BMB was graded MF-0 in 54% and MF-1 in 46% of control BMB; no difference was found in the proportion of MF-0/1 and 2/3 in BM0 compared to controls, but increased MF-2/3 was seen in BM-last compared to controls (p<0.001). At BM-last in patients dichotomized by MF-0/1 vs. MF-2/3, differences in hemoglobin levels (13.6 vs. 12.4 g/dl, respectively), absolute neutrophil counts (4.8 vs. 7 x109/L), platelet counts (92 vs. 123 x109/L), and LDH levels (212 vs. 219 U/L) were not significantly different.
Of the following 6 clinical factors: age, duration of disease, duration of treatment, splenectomy status, type and dose of agent; only age was significantly higher in patients with MF-2/3 as opposed to MF0/1 at time of BM-last [57 vs. 38 years; p=0.01]. There was a tendency toward longer duration of treatment in patients with MF-2/3 as compared to MF-0/1 (3.6 y vs. 2.7y; p=0.16).
Flow cytometric immunophenotyping of BMB in 89 examinations did not reveal emergence of clonal abnormalities. Cytogenetic analysis in 72 BMBs did not show any clonal karyotypic abnormalities.
This large single center experience indicates that TRAs induce some degree of MF as supported by: 1) decreasing fraction of MF-0 after initiation of TRA, 2) decreasing fraction of MF-0/1 (normal grades of MF) in subsequent on-treatment BMBs, 3) increasing fraction of MF-2/3 (pathological grades) in patients with multiple on-treatment BMBs. Only older age was associated with higher grades of fibrosis. However, MF remained stable in most patients within the range found in normal individuals. Higher grades of MF (MF-2/3) observed in some patients were not clinically significant based on peripheral blood counts. Overall, since a number of patients developed MF-2 and even MF-3, this suggests a risk of progressive fibrosis in approximately 20% of patients. No neoplastic immunophenotypic or karyotypic abnormalities emerged during treatment with TRAs. Annual or bi-annual follow-up with BMB should be carefully considered in TRA-treated patients. Discontinuation of TRA should be encouraged in those who develop/progress to MF-3 and possibly even MF-2 to avoid potential further progression of MF
Bussel:Amgen: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Genzyme: Research Funding; IgG of America: Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Eisai: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Symphogen: Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal