Abstract
Glycoprotein (GP)Ib-IX complex is critical to hemostasis and thrombosis. Its proper assembly is closely correlated with its surface expression level and requires cooperative interactions among extracellular and transmembrane domains of GPIbα, GPIbβ, and GPIX subunits. Two interfaces have been identified between 2 GPIbβ and GPIX extracellular domains.
To understand how extracellular domains interact in GPIb-IX.
The GPIbβ extracellular domain (GPIbβE) or the GPIX counterpart (GPIXE) in GPIb-IX was replaced simultaneously or individually with a GPIbβE/GPIXE chimera called GPIbβEabc (Fig1), and the effect of domain replacement on assembly and expression of the receptor complex in transiently transfected Chinese hamster ovary cells was analyzed.
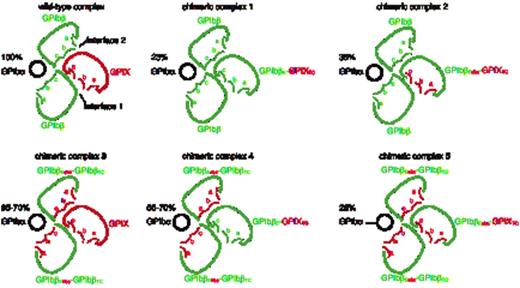
The figure above shows the top-view schematic map of extracellular domains of each transfected complex in experiments. Black circles represent GPIbα, green color represents the extracellular domain of GPIbβ and red color represents the extracellular domain of GPIX or convex loops derived from GPIX extracellular domain. The letters, a, b and c, mark the convex loops in the extracellular domains of GPIbβ and GPIX. Interface 1 and interface 2 between extracellular domains of GPIbβ and GPIX are noted in the first panel. The percentage of GPIbα fluorescence in each transfected complex versus that in transfected wild-type GPIb-IX complex is noted above each GPIbα subunit.
The figure above shows the top-view schematic map of extracellular domains of each transfected complex in experiments. Black circles represent GPIbα, green color represents the extracellular domain of GPIbβ and red color represents the extracellular domain of GPIX or convex loops derived from GPIX extracellular domain. The letters, a, b and c, mark the convex loops in the extracellular domains of GPIbβ and GPIX. Interface 1 and interface 2 between extracellular domains of GPIbβ and GPIX are noted in the first panel. The percentage of GPIbα fluorescence in each transfected complex versus that in transfected wild-type GPIb-IX complex is noted above each GPIbα subunit.
Five chimeric complexes, along with wild-type complex shown in Fig1, are characterized. All five chimeric complexes formed wild-type-like GPIbα-GPIbβ disulfide bonds, indicating that the interactions between extracellular domains do not affect formation of the GPIbα-GPIbβ disulfide bonds. Replacing GPIXE with GPIbβEabc in GPIb-IX retained interface 1, but not interface 2. While this domain replacement retained the ability of GPIbβ to aid expression of GPIX and the overall complex organization, the reciprocal enhancing effect of GPIX on expression of GPIbβ and GPIbα was lost. However, when the extracellular domain in GPIbβ was replaced with GPIbβEabc, surface expression level of each subunit in chimeric complex 3 and chimeric complex 4 expressing cells, was slightly lower than those in wild-type complex expressing cells, but significantly higher than those in other chimeric complexes expressing cells, indicating that chimeric complexes 3 and 4 share the same stability, but adopt different conformations. After replacing the ectodomains in both GPIbβ and GPIX with GPIbβEabc, the expression levels of GPIbα and associated subunits in chimeric complex 5 expressing cells were as low as those in chimeric complex 1 expressing cells, despite the different ability of GPIbβEabc and GPIbβE to self-associate.
The results demonstrate the importance of the association between GPIbβ and GPIX extracellular domains for assembly and efficient expression of GPIb-IX. In particular, interface 2 is critical for the stability of GPIbβ in the complex. However, not all the effects of extracellular domain replacement on GPIb-IX expression can be explained by the two separate interfaces, suggesting that GPIbβ and GPIX extracellular domains are malleable to accommodate and propagate potential conformational changes therein. This finding has significant implication on the molecular mechanism underlying the GPIb-IX-mediated signaling in platelets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal