Abstract
Microglia are the primary immune effector cells in the central nervous system. These cells originate from mesodermal monocytes that invade the developing central nervous system when the blood-brain barrier is not fully formed. Recently, microglial turnover has been suggested to occur by replenishment of progenitor cells from circulating blood or bone marrow (BM) (Simard and Rivest, FASEB J., 18, 998-1000, 2004) and by proliferation of resident microglia. We previously demonstrated that microglia phagocytose amyloid-fÀ (AfÀ) peptides (Takata et al, J. Biol. Chem., 285, 40180-40191, 2010), which are the principal causative factor in Alzheimer's disease (AD). We further showed that transplantation of exogenous microglia into rat brain effectively reduces deposition of AfÀ in the brain (Takata et al, FEBS. Lett., 581, 475-478, 2007). Thus, strategies to treat AD using microglia are eagerly awaited. However, preparation of microglia from human brain is challenging. This may be overcome by differentiating microglia from hematopoietic stem/progenitor cells. In this study, we treated mouse and human BM cells with macrophage-colony stimulating factor (M-CSF) to generate microglia-like monocytic cells. The ability of these cells to phagocytose AfÀ and their effect on phagocytosis by resident microglia were analyzed.
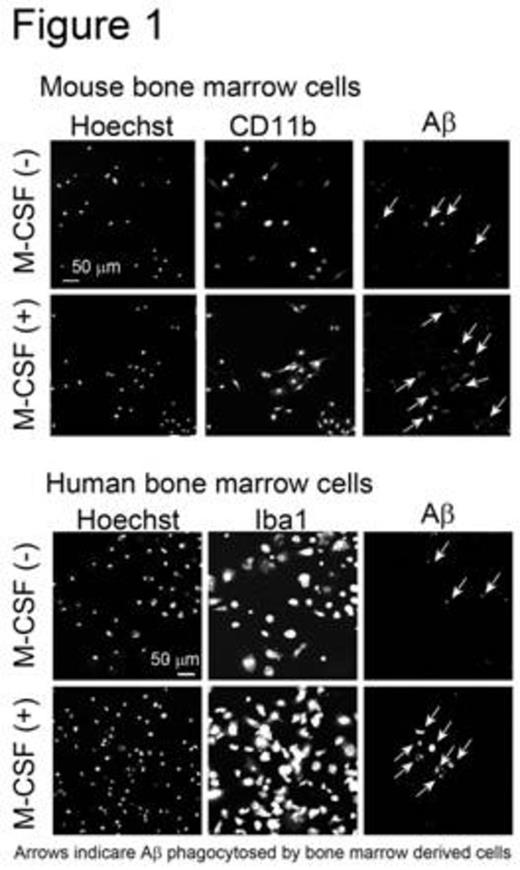
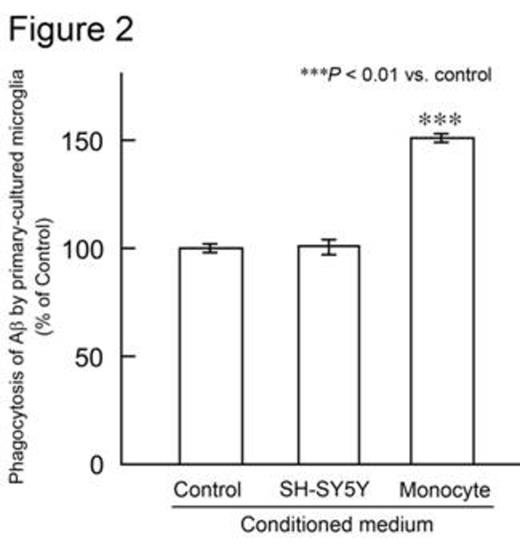
The femurs and tibias of male C57BL/6 mice were flushed to harvest BM cells. Human CD34+ BM cells were purchased from Lonza (Basel, Switzerland). BM cells were cultured in minimum essential medium-f¿ in the presence or absence of human M-CSF. After 0, 3, 7, or 14 days of culture, the number of adherent cells was counted using a cell counter plate. Cell differentiation was analyzed by flow cytometry and confocal laser microscopy. M-CSF treatment markedly increased the number of adherent mouse and human cells. Adherent cells derived from mouse BM cells expressed monocytic/microglial markers, such as CD11b, CD68, and ionized calcium-binding adapter molecule 1. Furthermore, M-CSF treatment increased the number of cells expressing these markers. These data indicate that BM cells differentiate into microglia-like monocytic cells when treated with M-CSF. Next, the phagocytic abilities of microglia-like cells derived from mouse and human BM cells were examined. Adherent cells were treated with synthetic human AfÀ1-42 peptide. Microglia-like cells phagocytosed AfÀ1-42, as observed by confocal laser microscopy. Moreover, M-CSF treatment increased the number of cells that phagocytosed AfÀ1-42 (Figure 1). Subsequently, we investigated whether these microglia-like cells stimulate primary-cultured resident mouse microglia in culture. Adherent cells were obtained by treating mouse BM cells with M-CSF, and were then incubated in medium lacking M-CSF. The conditioned medium was collected and this, along with the AfÀ1-42 peptide, was added to primary-cultured microglia. The amount of AfÀ the microglia phagocytosed was analyzed using ELISA. Interestingly, conditioned medium from mouse BM-derived cells, but not from human neuroblastoma SH-SY5Y cells, significantly increased phagocytosis of AfÀ1-42 by primary-cultured mouse microglia (Figure 2). These data suggest that BM-derived microglia-like cells secrete humoral factors that stimulate resident mouse microglia. The identification of these factors is ongoing.
We demonstrated that M-CSF treatment effectively induces differentiation of BM cells into microglia-like monocytic cells that can phagocytose AfÀ. Furthermore, this is the first study to show that microglia-like monocytic cells secrete humoral factors that facilitate phagocytosis of AfÀ by resident microglia. Microglia-like monocytic cells prepared from BM cells by using M-CSF could be used as a disease-modifying therapeutic strategy for AD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal