Abstract
Patients (pts) with MM often present with varying degrees of renal insufficiency (RI) which can cause significant morbidity. In younger pts with MM, high-dose chemotherapy with autologous stem cell transplantation (ASCT) has been shown to enhance remission and possibly improve survival. In pts with RI there has been concern about the safety and efficacy of proceeding to ASCT. At our center, ASCT has been routinely offered to pts with RI. Herein we report the results of MM pts with RI who received ASCT since 2000.
Outcomes of 50 MM pts with RI were compared to 100 matched ASCT recipients. The matched transplant pts studied were similar with respect to subject-, disease-, and transplant-related characteristics. All pts met standard eligibility criteria for ASCT and pts with RI had a serum creatinine (Cr) level of >2 mg/dL (average Cr = 6.07 with a range of 2 to 11.79 mg/dL), at the time of ASCT. 14 of the 50 pts were on dialysis at the time of ASCT. All patients had active MM requiring therapy and all pts had evidence of stable disease or treatment response prior to transplant. For pts with RI, the average age at transplant was 56 years (range 33 to 69 yrs) and the median time between diagnosis and ASCT was 302 days. The preparative therapy consisted of single agent Melphalan, given on Day (-3) or (-2) with a median administered dose of 140 mg/m2 (range 100-200 mg/m2) while the dose for the matched patients was 200mg/m2. All pts were hospitalized for ASCT and received standard supportive care; >2 x 10e6/Kg CD34(+) cells, G-CSF, transfusions as necessary and appropriate anti-infectives.
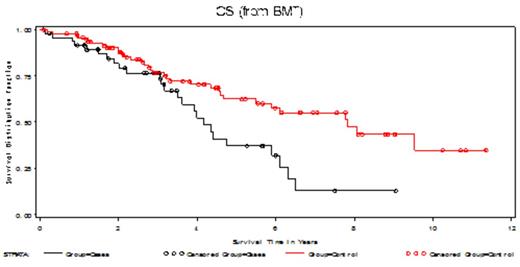
The 30-day and 100-day treatment related mortality (TRM) rates for both the patients with RI and matches were 0%. Amongst the patients with RI, 4 patients required ICU care and the average duration of hospitalization was 23.36 days (range: 14 - 48 days) and duration of hospitalization for matches 19.4 days (range: 11-58 days). Time to ANC > 0.5 x10e9/L was 12 days (range 10-21 days) and 12 days (range 10-22 days) in the RI and matched cohort respectively. Time to platelets > 20 x 10e9/L was 11 days (range: 6-22 days) and 14 days (range 8-51days) in the RI and matched cohort respectively. 32% of pts with RI were in very good partial response (VGPR)+ complete remission (CR) of which 12% were in CR at ASCT. After ASCT, 50% of pts achieved a VGPR+CR of which 34% pts achieved a CR. The mean overall survival (OS) for patients with RI was 4.2 yrs and for controls was 6.5 yrs [Figure 1. HR 2.036, 95% CI (1.204-3.443) p=0.0084].
Kaplan-Meier OS curve of pts with RI (black) and matched controls (red).
ASCT is a safe and effective treatment option for MM pts with RI including those on dialysis with engraftment characteristics similar to pts without RI.
Wolf:Onyx: Honoraria; Millenium: Honoraria; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal