Abstract
Progression free and overall survival (PFS/OS) for Multiple Myeloma (MM) have improved over the past 20 years largely as a result of ASCT as well as novel conventional dose therapeutic agents. However, since the 1990s improvements in PFS and OS due to ASCT have improved minimally due to continued reliance on single agent melphalan (MEL). In order to improve PFS and OS, better transplant regimens should be investigated. The combination of Busulfan (BU) and MEL delivers better PFS compared to MEL alone (Lahuerta, et al) with similar toxicity rates to MEL alone. Furthermore, in vitro and in vivo studies indicate synergy between MEL and proteasome inhibitors such as Botezomib (BTZ). Superior response rates and PFS were seen when BTZ is combined with MEL when compared with historical controls using MEL in a recent IFM study. (Roussel et al. Blood 2010). We hypothesize that IV BU and MEL followed by BTZ (BuMelVel) could be an effective preparative regimen with acceptable toxicity for patients with MM.
Between July 2009 and June 2013 57 patients with Multiple Myeloma who had already undergone induction therapy and were eligible for ASCT were enrolled. Patients received IV BU administered as a daily intravenous infusion for a total of 4 days with the first 2 days (day -6, -5) at fixed dose of 130 mg/m2 over 3 hours and the subsequent 2 doses (day -4, -3) adjusted to achieve a target area under the concentration-time curve (AUC) total of 20,000 mM* min. Pharmacokinetic (PK) analysis performed after the first dose of IV Bu was used to determine Bu AUC and individualized Bu PK-directed dosing for later doses (d-4 and -3). MEL was administered at 140 mg/ m2 IV over 15-30 minutes on D-2. BTZ 1.6 mg/m2 was administered IV push on D-1. Palifermin was given for mucoprotection at a dose of 6.25 mg IV for two consecutive days before the first busulfan dose (days -8 and -7). A third dose of 6.25 mg was give on day 0 after stem cell transplantation.
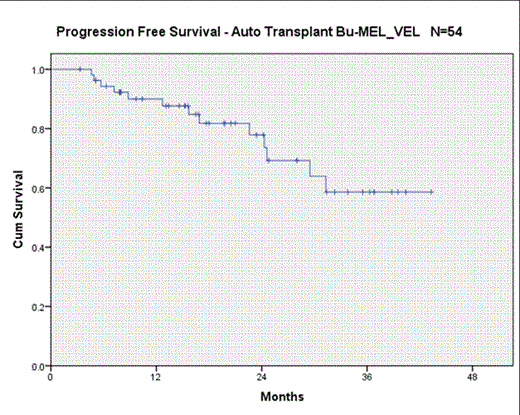
Of the 57 patients enrolled 56 are evaluable for toxicity and 54 for response at D +100. Median age is 61 (31 - 72). 49 % of patients had received ≥ 2 induction regimens (range 1-4) and 69% were DS stage III. 38% of patients had achieved at least a VGPR after induction with 9% of those achieving a CR. After transplantation, 70% of patients had at least a VGPR including 37% CR or sCR. All but 4 patients had a PR or better to induction therapy (three had stable and one progressive disease). The most common grade ≥ 3 toxicities were neutropenic fever (n = 42) and mucositis (n=21). No VOD or treatment related deaths at D+100 were observed and all patients engrafted. Median time to engraftment was 10 days (range 10-12) and median hospital stay was 20 days ( 15-31). Median PFS at 2 years was 78%.
BuMelVel is an effective novel preparative regimen with ≥ VGPR and CR/sCR of 70% and 37% respectively which compare favorably to responses previously reported with MEL 200 alone (43% and 11%, respectively) by Roussel et al. At the time of reporting, 12 patients had relapsed; 2 and 3-year PFS rates were 78% and 60% respectively. The regimen of BuMelVel is well tolerated and may lead to improvements in PFS and OS in patients with multiple myeloma.
Rodriguez:Otsuka: Research Funding; Millennium: Research Funding, Speakers Bureau; Celgene: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal