Abstract
Ex vivo T-cell depleted (TCD) allogeneic hematopoietic stem cell transplants (HCT) in patients with acute myeloid leukemia in complete remission (CR)lead to survival and relapse rates comparable to those with unmodified grafts, but with significantly less graft versus host disease (GVHD). TCD-HCT is also effective in patients with acute lymphoblastic leukemia (ALL), however it is not known how outcomes compare with those of patients receiving conventional grafts. In this study we compared the outcomes of patients receiving TCD-HCT at Memorial Sloan-Kettering Cancer Center (MSKCC) with patients receiving conventional grafts at MD Anderson Cancer Center (MDACC) for ALL in first or second CR.
Patients 18 years of age or older, undergoing HCT between 2000 and 2010 were included in this retrospective analysis. 52 patients at MSKCC received TCD grafts and 129 patients at MDACC received conventional grafts. All patients at MSKCC received myeloablative HCT conditioning (MAC). No GVHD prophylaxis was given. At MDACC, 115 patients received MAC and 14 received reduced intensity conditioning; GVHD prophylaxis consisted of tacrolimus and mini-dose methotrexate, plus antithymocyte globulin for total dose 4 mg/kg for unrelated donors.
Patient characteristics and outcomes are summarized in the Table. A greater percentage of patients in the conventional graft group received transplants from a matched related donor and from bone marrow as a stem cell source. Patients in the TCD group almost exclusively received grafts from peripheral blood.
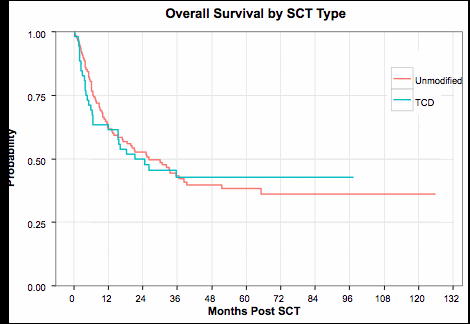
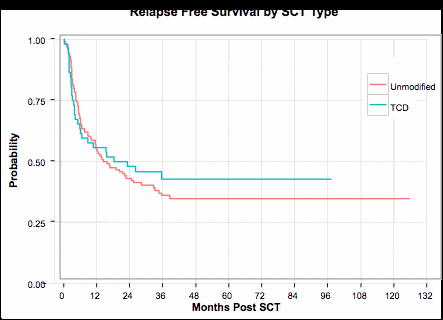
A significant difference in acute GVHD incidence was observed between the TCD and conventional groups, with 100-day cumulative incidence estimates of 17.3% and 40.3%, respectively (Gray's test p-value=0.007). A significant difference was also observed in chronic GVHD rates, with three-year estimates of 13.5% and 34.8% for the TCD and unmodified groups, respectively (Gray's test p-value=0.004). There was no statistical difference between TCD and unmodified groups with regards to OS (log-rank p-value=0.89, 3 year estimates 42.6% vs. 43.2%) or RFS (log-rank p-value=0.64, 3 year estimates 42.8% and 36.0%). The NRM at day 100, 1 year and 3 years were 15.4%, 26.9% and 37.8% in the TCD group and 10.1%, 23.5% and 28% in the unmodified group (Gray's test p-value=0.22). There was a lower relapse rate among patients who received TCD versus unmodified grafts (3 year estimates 19.4% vs. 36.0%, Gray's test p-value=0.05). Age >50 and was the only factor associated with inferior RFS (HR=1.74, p=0.01) and OS (HR=1.85, p=0.006).
HCT is an effective treatment modality for patients with ALL with favorable results with either conventional or TCD allografts. Although the relapse rate was significantly lower after TCD-HCT, this would need to be confirmed in a prospective study. While 3-year OS rates are similar in both groups, TCD effectively reduces the rate of both acute and chronic GVHD.
*: Median (range)
* *: P-values were estimated using a chi-square test or Fisher's exact test for categorical variables and a Wilcoxon rank-sum test for continuous variables. OS and RFS were compared using a logrank statistics, while NRM, aGVHD and cGHVD were compared using Gray's test.
^: Good risk: Hyperdiploidy, del(9p); poor risk: Ph+, t(4;11), t(8;14); complex karyotype (≥5 abnormalities), hypodiploidy/near triploidy; standard risk: other.
Probabilities of overall (A) and relapse-free (B) survival in recipients of T cell depleted (TCD) and unmodified grafts.
Probabilities of overall (A) and relapse-free (B) survival in recipients of T cell depleted (TCD) and unmodified grafts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal