Abstract
In adults, ITP (characterized by platelet counts <100x109/L) is typically chronic, with remission reported infrequently ≥3 y post-diagnosis (Sailer, Haematol 2006). The TPO-mimetic romiplostim increases platelet counts and reduces use of concomitant ITP medications in chronic ITP. While often perceived as a long-term treatment, dose adjustment rules allow romiplostim to be discontinued when hemostatic platelet counts are reached, as reported in Amgen trials (pivotal trials, 2011 EHA, 2011 ASH) and case reports.
Data from 8 romiplostim trials (4 phase 3, 2 single-arm, and 2 extension trials) were examined for cases in which patients receiving romiplostim subsequently had ≥26 consecutive wks of platelet counts ≥50x109/L without romiplostim or any other ITP medications. The number of evaluable patients could not be calculated as many trials had dosing rules that did not allow for the reduction of romiplostim in patients with platelet counts <400x109/L (making remission unlikely), treatment durations varied by study, and follow-up data were not always available.
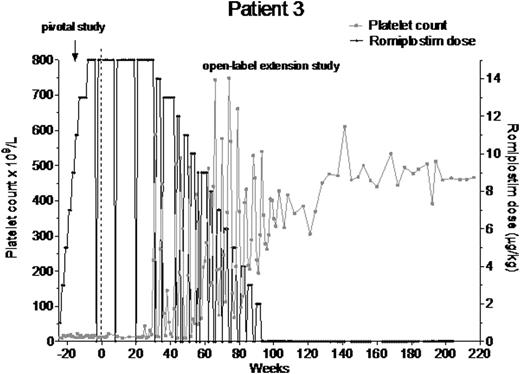
Twenty-seven patients had ≥26 wk of platelet counts ≥50x109/L without romiplostim or any other ITP medications (Table; data from 4 of these patients were presented at ASH 2011). These patients had characteristics of median (Q1, Q3) time since ITP diagnosis of 2.1 (0.5, 4.2) y, with 17/27 having ITP >1 y, mean (SD) baseline platelet count of 20.9 (15.41) x109/L, median (Q1, Q3) age of 49.0 (36.0, 67.0) y, and mean (SD) maximum dose prior to remission of 4.6 (3.6) µg/kg. Of the 27 patients, 12 (44%) were splenectomized at baseline and 15 (56%) were male. Patients had from 40 to 276 cumulative wks of romiplostim with doses ranging from 0.1 to 4.3 µg/kg. Prior to romiplostim treatment, minimum platelet counts ranged from 1-37x109/L and individual average platelet counts ranged from 1-152x109/L. On treatment, minimum (1-182x109/L) and average (121-654x109/L) platelet counts ranged widely. The median (Q1, Q3) time to remission was 7.1 (1.9, 12.7) months. Only 3 patients (3, 6, 15) had bleeding of grade 3; the remainder had either bleeding of grade 1 or 2 or none.
| Pt . | Age (y) . | Sex . | Duration of ITP (y) . | Past ITP Rx . | Splenect Before Study? . | BL Plt Count (x109/L) . | Wks of Romip . | Mean Romip Dose (µg/kg) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | 3.07 | Steroid, Anti-D, IVIg | N | 26 | 267 | 3.0 |
| 2 | 51 | F | 1.16 | Steroid, Anti-D | N | 49 | 276 | 0.6 |
| 3 | 29 | M | 5.5 | Steroid, Anti-D, IVIg, Vincrist/blast, Danazol, Cyclophosp, Ritux, Stem cell, Plt | Y | 13 | 240 | 4.3 |
| 4 | 49 | F | 4.2 | Steroid, Anti-D, IVIg | Y | 26.3 | 138 | 0.4 |
| 5 | 78 | F | 3.3 | Steroid, Anti-D, IVIg, Danazol, Azathioprine, Ritux | N | 24.3 | 239 | 0.7 |
| 6 | 37 | F | 0.8 | Steroid, IVIg | N | 38 | 187 | 2.1 |
| 7 | 46 | M | 2.13 | N/A | N | 27 | 91 | 0.1 |
| 8 | 25 | F | 3.26 | N/A | Y | 9 | 59 | 0.5 |
| 9 | 73 | M | 2.36 | N/A | Y | 10 | 127 | 1.2 |
| 10 | 35 | M | 0.15 | N/A | Y | 10 | 40 | 0.2 |
| 11 | 46 | M | 0.48 | N/A | Y | 15 | 80 | 1.6 |
| 12 | 58 | M | 22.85 | N/A | Y | 15 | 74 | 1.6 |
| 13 | 77 | M | 3.29 | N/A | N | 4 | 57 | 0.9 |
| 14 | 51 | M | 1.28 | N/A | Y | 5 | 57 | 2.3 |
| 15 | 36 | F | 5.34 | N/A | Y | 35 | 108 | 3.4 |
| 16 | 36 | F | 1.96 | N/A | Y | 9.5 | 42 | 0.1 |
| 17 | 67 | M | 5 | N/A | Y | 17 | 46 | 0.2 |
| 18 | 62 | F | 0.23 | Steroid, Anti-D, IVIg | N | 1 | 51 | 0.3 |
| 19 | 42 | M | 0.11 | Steroid, IVIg | N | 12 | 131 | 0.8 |
| 20 | 23 | M | 0.04 | Steroid | N | 13 | 52 | 0.3 |
| 21 | 73 | M | 6.49 | Steroid, IVIg, Ritux, Cyclosporine | N | 6 | 52 | 1.0 |
| 22 | 45 | F | 0.57 | Steroid, IVIg | N | 34 | 117 | 0.8 |
| 23 | 72 | F | 0.45 | Steroid, IVIg | N | 9 | 135 | 1.1 |
| 24 | 74 | M | 0.1 | Steroid, IVIg | N | 65 | 51 | 0.1 |
| 25 | 54 | M | 0.05 | Steroid, IVIg | N | 30 | 54 | 0.0 |
| 26 | 45 | F | 26.96 | Steroid, IVIg, Danazol, Ritux | Y | 43 | 42 | 0.2 |
| 27 | 32 | M | 2.05 | Steroid | N | 18.7 | 45 | 0.3 |
| Pt . | Age (y) . | Sex . | Duration of ITP (y) . | Past ITP Rx . | Splenect Before Study? . | BL Plt Count (x109/L) . | Wks of Romip . | Mean Romip Dose (µg/kg) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | 3.07 | Steroid, Anti-D, IVIg | N | 26 | 267 | 3.0 |
| 2 | 51 | F | 1.16 | Steroid, Anti-D | N | 49 | 276 | 0.6 |
| 3 | 29 | M | 5.5 | Steroid, Anti-D, IVIg, Vincrist/blast, Danazol, Cyclophosp, Ritux, Stem cell, Plt | Y | 13 | 240 | 4.3 |
| 4 | 49 | F | 4.2 | Steroid, Anti-D, IVIg | Y | 26.3 | 138 | 0.4 |
| 5 | 78 | F | 3.3 | Steroid, Anti-D, IVIg, Danazol, Azathioprine, Ritux | N | 24.3 | 239 | 0.7 |
| 6 | 37 | F | 0.8 | Steroid, IVIg | N | 38 | 187 | 2.1 |
| 7 | 46 | M | 2.13 | N/A | N | 27 | 91 | 0.1 |
| 8 | 25 | F | 3.26 | N/A | Y | 9 | 59 | 0.5 |
| 9 | 73 | M | 2.36 | N/A | Y | 10 | 127 | 1.2 |
| 10 | 35 | M | 0.15 | N/A | Y | 10 | 40 | 0.2 |
| 11 | 46 | M | 0.48 | N/A | Y | 15 | 80 | 1.6 |
| 12 | 58 | M | 22.85 | N/A | Y | 15 | 74 | 1.6 |
| 13 | 77 | M | 3.29 | N/A | N | 4 | 57 | 0.9 |
| 14 | 51 | M | 1.28 | N/A | Y | 5 | 57 | 2.3 |
| 15 | 36 | F | 5.34 | N/A | Y | 35 | 108 | 3.4 |
| 16 | 36 | F | 1.96 | N/A | Y | 9.5 | 42 | 0.1 |
| 17 | 67 | M | 5 | N/A | Y | 17 | 46 | 0.2 |
| 18 | 62 | F | 0.23 | Steroid, Anti-D, IVIg | N | 1 | 51 | 0.3 |
| 19 | 42 | M | 0.11 | Steroid, IVIg | N | 12 | 131 | 0.8 |
| 20 | 23 | M | 0.04 | Steroid | N | 13 | 52 | 0.3 |
| 21 | 73 | M | 6.49 | Steroid, IVIg, Ritux, Cyclosporine | N | 6 | 52 | 1.0 |
| 22 | 45 | F | 0.57 | Steroid, IVIg | N | 34 | 117 | 0.8 |
| 23 | 72 | F | 0.45 | Steroid, IVIg | N | 9 | 135 | 1.1 |
| 24 | 74 | M | 0.1 | Steroid, IVIg | N | 65 | 51 | 0.1 |
| 25 | 54 | M | 0.05 | Steroid, IVIg | N | 30 | 54 | 0.0 |
| 26 | 45 | F | 26.96 | Steroid, IVIg, Danazol, Ritux | Y | 43 | 42 | 0.2 |
| 27 | 32 | M | 2.05 | Steroid | N | 18.7 | 45 | 0.3 |
BL, baseline; Cyclophos, cyclophosphamide; F, female; IVIg, immunoglobulins; M, male; Plt, platelet; Pt, patient; Ritux, rituximab; Romip, romiplostim; Rx, treatment; Splenect, splenectomy; Steroid, corticosteroid; Vincrist/blast, vincristine/vinblastine. N/A = data unavailable.
The patient population who entered clinical remission (platelet counts ≥50x109/L for 26 wks) after treatment with romiplostim generally had: ITP of less than 5 y duration, a wide range of ages (23-78 y), about equal proportions of men and women, were as likely to be splenectomized as not, and no significant bleeding. Therefore, it is difficult to predict which patients would have this response. Reporting of these cases was not predefined, so a prospective assessment, such as the phase 2 single-arm study currently underway (NCT01143038), will provide a more comprehensive evaluation of remission with romiplostim. Potential mechanisms for the phenomenon of remission, both in early and late stages of ITP, may involve T-regulatory cell function, increased numbers/activity of natural killer T-cells, or increased B-regulatory cell activity.
Bussel:Amgen: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Genzyme: Research Funding; IgG of America: Research Funding; GSK: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Ligand: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Immunomedics: Research Funding; Eisai: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Portola: Consultancy. Wang:Amgen: Employment, Equity Ownership. Eisen:Amgen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal