Abstract
Lenalidomide is an immunomodulatory drug that is active in newly diagnosed as well as relapsed multiple myeloma (MM). The goal of this study is to describe patients with newly diagnosed MM remaining on lenalidomide for more than 3 years (long term responders) as well as patients that after discontinuing lenalidomide were able to maintain disease control while only on observation (LenO group).
We retrospectively reviewed 283 patients with newly diagnosed MM that were treated with lenalidomide between January 2003 and January 2011. We excluded patients that underwent early autologous stem cell transplant (n=102) or had less than 3 years of follow-up (n=6), leaving 175 patients for the current analysis.
Long term responders
Thirty-three patients (19%) received lenalidomide for more than 3 years. When compared to patients receiving lenalidomide for less than 3 years, at baseline, long term responders were more likely to have standard risk disease (64% versus 44%, p=0.03) and less likely to have high risk disease (18% versus 4%, p=0.04). They were more likely to have achieved a deeper response (VGPR versus PR, p<0.001) and had a longer median time to best response (6 versus 4 months, p<0.001). When considering the 12 patients who received lenalidomide for more than 5 years, this group was more likely to have achieved a deeper response (CR versus PR, p<0.0001) and had a longer median time to achieving best response (9 versus 4 months, p<0.0001) than those 163 patients who remained on lenalidomide for fewer than 5 years.
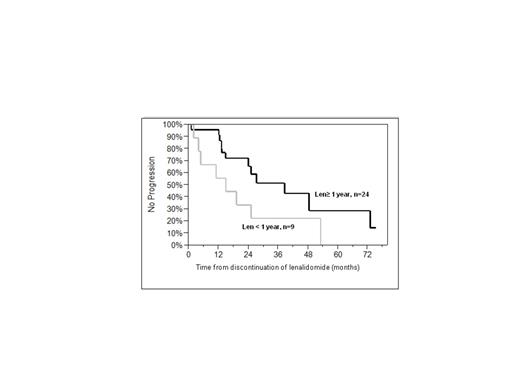
Thirty-three patients (19%) discontinued lenalidomide for reasons other than progression and were observed without receiving further treatment (LenO group). Prior to moving to observation, five patients had received lenalidomide for more than three years. Indications for stopping in the LenO group included: prolonged disease stability (n= 20); and toxicity (n=13). The only differences in baseline characteristics between the LenO group and patients that were not observed off any treatment was depth of response, with 61% (n=20) of LenO in VGPR or better versus 23% (n=23) in the remainder, p=0.0003. Median PFS from the time of discontinuing lenalidomide was significantly longer in those patients who took lenalidomide for more than 1 year (n=24) when compared to patients taking it for less than one year (n=9) (median PFS off therapy was 38.5 months versus, 14.9 months log rank 0.08; Wilcoxon p<0.05), figure 1a; PFS for those treated for 1-2 years (n=11) as compared to 2 years or greater (n= 13) were comparable to each other (data not shown). Among those taking lenalidomide for at least a year, PFS from time of discontinuing lenalidomide was superior in patients who had achieved a VGPR/CR (n=20) as compared to those who achieved only a PR (n=13) (Median 48.4 months versus 14.8 months, log-rank p<0.05; Wilcoxon p=0.01), figure 1b.
Progression free survival (PFS) of patients in the LenO group according to (a) lenalidomide duration and (b) depth of response
Progression free survival (PFS) of patients in the LenO group according to (a) lenalidomide duration and (b) depth of response
Approximately one out of five patients with newly diagnosed MM can achieve responses lasting more than three years while on treatment with lenalidomide. Patients with standard risk FISH and those achieving at least VGPR are more likely be long-term responders. Furthermore, long-term responders were more likely to be slow responders. We also demonstrate that suspension of lenalidomide therapy after 1 year among those patients achieving a VGPR or better can result in long-term disease control and can be considered as a therapeutic strategy.
Kumar:Celgene: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Onyx: Consultancy, Research Funding. Gertz:Celgene: Honoraria. Lacy:Celgene: Honoraria. Dispenzieri:Celgene: Research dollars Other.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal