Abstract
We piloted an induction regimen of cyclophosphamide, bortezomib and dexamethasone (CyBorD)(CBD) in 2006 and showed rapid and deep responses after 4 cycles in patients with newly diagnosed multiple myeloma (MM).1 We subsequently reported equal responses and less toxicity with weekly bortezomib dosing.2 Triplet therapy has since become common in both standard and high risk patients and no other triplet combination has shown results superior to CyBorD.3 We report long-term f/u data for all 63 patients treated on both cohorts at MCA and PMH.
Sixty-three patients with newly diagnosed and symptomatic myeloma were enrolled between 2006 and 2008 on a phase II trial of weekly oral cyclophosphamide 300mg/m2, IV bortezomib 1.3 mg/m2 d1, 4, 8, 11 (Cohort1) or 1.5mg/m2 d1, 8, 15, 22 (Cohort2) and dexamethasone in high dose (Cohort 1) or high dose for 2 cycles, then low dose for 2 cycles (Cohort2). Cycles were 28 days and patients received prophylactic acyclovir and a quinolone antibiotic. Response after 4 cycles was the primary goal and secondary goals were PFS, OS and toxicity of this regimen. Patients were stratified to high risk or standard risk by mSMART criteria.
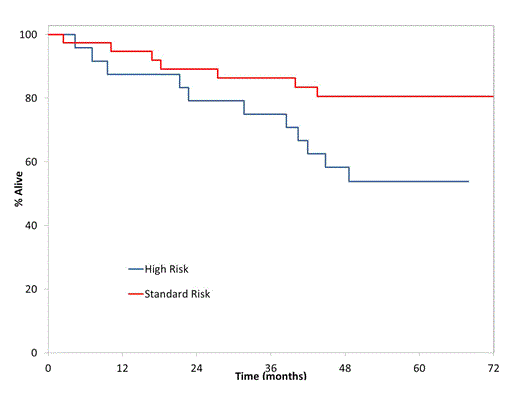
The overall response for all patients (n=63) was 89% with 62% VGPR or better. The 5 year PFS and OS rates were 42% (95%CI: 31-57) and 70% (95%CI: 59-82) for the entire group (Figure 1). Twenty-four of the 63 patients were high risk (38%). Patients considered high risk had a lower 5 year PFS (33% (95%CI: 19-59) vs 48% (95%CI: 33-69)) and OS rate (54% (95%CI: 37-78) vs 81% (95%CI: 69-95) compared to standard risk. Most patients underwent auto stem cell transplant after completing the induction trial and most did not utilize maintenance therapy post SCT.
Early and deep responses are needed for long-term disease control in MM. CyBorD maximizes responses and allows for stem cell collection and transplantation which can further consolidate response and improve outcome. This combination is more cost effective than other triplet options while yielding equivalent responses and preserving the opportunity for future IMiD therapy. CyBorD employed in a once weekly schedule is not associated with thrombosis or severe neuropathy. The result is excellent survival for patients with standard risk multiple myeloma. Eighty-one percent of standard risk patients are alive at 5 years. High risk MM patients respond well but survival data confirm the need for ongoing consolidation and or maintenance therapy. CyBorD induction should be considered a standard regimen for transplant-eligible patients with newly diagnosed MM.
Ref
1 Leukemia 2009; 23(7):1337-1341
2 Blood 2010; 115(16):3416-3417
3 Blood 2012; 119(19):4375-4382
Kaplan Meier Survival Curves for All Patients
Kaplan Meier Survival Curves by mSMART Risk
Reeder:Novartis: Research Funding; Celgene: Research Funding; Millennium: Research Funding. Chen:Celgene: Consultancy, Honoraria, Research Funding. Fonseca:millennium: Consultancy; amgen: Consultancy; Binding site: Consultancy; onyx: Consultancy; medtronic: Consultancy; Genzyme: Consultancy; Otsuka: Consultancy; Celgene: Consultancy; lilly: Consultancy; Onyx: Research Funding; cylene: Research Funding. Bergsagel:Onyx: Consultancy. Tiedemann:Celgene: Honoraria; Janssen: Honoraria. Stewart:Onyx: Consultancy, Research Funding; Millennium: Honoraria, Research Funding; Celgene: Honoraria; BMS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal