Abstract
Bortezomib-based therapies are among the standards of care for the treatment of MM in the first-line and relapsed settings. In treatment-naïve patients, the triplet regimens VCD and VRD have demonstrated substantial efficacy in terms of tumor response and tolerability in phase 2 clinical trials. While both therapies have been used for first-line treatment of MM in clinical practice, the costs and adverse events (AEs) associated with these regimens differ considerably, and there are limited data comparing these regimens in routine clinical settings. This two-center, US-based, retrospective, observational study compared the effectiveness and safety of VCD and VRD as initial treatment for MM in routine oncology clinical settings. The objectives were to assess outcomes and differences in safety profile with VCD and VRD.
Data for inclusion in the analysis were extracted, using a pre-specified case report form, from randomly selected medical charts of previously untreated MM patients aged ≥18 years who initiated VCD or VRD treatment at Mayo Clinic, Rochester, or within the US Oncology Network, from 2008 to 2013, and who had ≥2 clinic visits and ≥6 months of follow-up from first administration of VCD/VRD. The primary endpoint was best response to VCD/VRD treatment after 4 cycles and at the end of treatment. Secondary endpoints included progression-free survival (PFS), overall survival (OS), and rates of AEs. Response rates and rates of AEs were compared using chi-square tests. PFS and OS at 1 and 2 years were analyzed using Kaplan-Meier curves and log-rank tests; adjusted hazard ratios (HRs) were estimated from Cox models controlling for ISS stage III disease, stem-cell transplant (SCT), renal failure, cardiac disease, diabetes mellitus, and bone fractures.
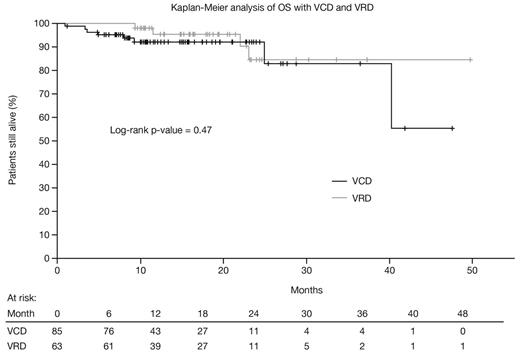
88 and 71 patients treated with VCD and VRD, respectively (45/43 and 45/26 from US Oncology/Mayo Clinic), were evaluated; 3 and 8 were treated in the context of a clinical trial and excluded from this analysis. Among the remaining 85 VCD and 63 VRD patients, respectively, mean (±SD) age was 62.5 (±9.8) and 62.0 (±10.2) years, 53% and 57% were male, 29% and 25% had ISS stage III disease, and 41% and 17% had renal failure (p=0.0021). Cytogenetic abnormalities (FISH) included t(4;14) in 5% and 3%, t(14;16) in 5% and 8%, and del17p in 5% and 10%. Treatment was administered in 21-/28-day cycles in 18%/82% of VCD patients and 87%/13% of VRD patients (p<0.0001). Mean (±SD) treatment duration was 5.0 (±3.5) and 5.3 (±4.3) months with VCD and VRD, respectively, corresponding to a mean (±SD) number of equivalent 21-day cycles of 6.3 (±3.2) and 6.2 (±3.8). 51% of VCD and 56% of VRD patients subsequently underwent a SCT. Best response rate after 4 cycles was 89% with VCD and 84% with VRD (p=0.34), and best response rate across treatment was 93% and 87%, respectively (p=0.25). With VCD and VRD, PFS rates were 74.8% and 77.3% at 1 year (p=0.81; adjusted HR 0.91 [95% CI 0.44–1.90], p=0.80) and 69.9% and 67.8% at 2 years (p=0.81; adjusted HR 0.95 [95% CI 0.48–1.92], p=0.90); OS rates (see figure) were 92.2% and 95.6% at 1 year (p=0.26; adjusted HR 1.59 [95% CI 0.28–9.02], p=0.60), and 92.2% and 84.6% at 2 years (p=0.70; adjusted HR 0.91 [95% CI 0.22–3.86], p=0.90). AEs reported at least once with VCD and VRD, respectively, were peripheral neuropathy (40%, 35%), anemia (38%, 35%), fatigue (38%, 29%), gastrointestinal toxicities (32%, 27%), infections (20%, 21%), thrombocytopenia (19%, 17%), neutropenia (18%, 13%), renal toxicities (15%, 14%), thrombosis (7%, 6%), and congestive heart failure (7%, 2%); there were no significant differences between treatment groups. Common AEs reported in at least 3 treatment cycles included fatigue (21%, 11%), peripheral neuropathy (13%, 17%), anemia (13%, 17%), and gastrointestinal toxicities (9%, 8%). All efficacy and safety findings were similar in analyses including the 11 patients treated in the context of clinical trials.
These data demonstrate comparable outcomes with initial therapy with VCD and VRD in terms of response, PFS, and OS, and similar safety profiles. Similar outcomes were seen despite the higher proportion of patients with renal insufficiency in the VCD arm, a feature associated with inferior outcomes. These findings, consistent with those from the randomized phase 2 EVOLUTION study, need to be taken in the context of the substantial treatment cost difference between the regimens.
Kumar:Genzyme: Research Funding; Novartis: Research Funding; Millennium: The Takeda Oncology Company: Research Funding; Celgene: Consultancy, Research Funding; Merck: Consultancy, Honoraria. Lacy:Celgene: Research Funding. Dispenzieri:Millennium: The Takeda Oncology Company: Research Funding; Pfizer: Research Funding; Celgene: Research Funding; Janssen: Research Funding. Duh:Millennium: The Takeda Oncology Company: Research Funding. Lafeuille:Millennium: The Takeda Oncology Company: Research Funding. Lefebvre:Millennium: The Takeda Oncology Company: Research Funding. Cheng:Millennium: The Takeda Oncology Company: Research Funding. Dea:Millennium: The Takeda Oncology Company: Research Funding. Yap:McKesson Specialty Health: Employment. Saravanan:McKesson Specialty Health: Employment. Pan:McKesson Specialty Health: Employment. Rembert:McKesson Specialty Health: Employment. Dhanda:McKesson Specialty Health: Employment. Niculescu:Millennium: The Takeda Oncology Company: Employment. Ba-Mancini:Millennium: The Takeda Oncology Company: Employment. Ma:Millennium: The Takeda Oncology Company: Employment. Quick:Millennium: The Takeda Oncology Company: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal