With the introduction of novel agents, survival in multiple myeloma has successively increased in the past decade. This improved survival increases the risk for late complications. Patients continue to receive melphalan therapy as a standard induction for those over the age of 65. Lenalidomide is considered a standard component of induction therapy in the United States for patients who are transplant candidates. Stem cell transplantation remains the standard of care for patients eligible to receive it. As a consequence of exposure to lenalidomide, stem cell transplant, and melphalan, the risk of late myelodysplasia and acute leukemia remains significant. We reviewed the Mayo Clinic experience with therapy-induced myelodysplasia beginning with the advent of novel agents.
All patients that carry the diagnosis of multiple myeloma between January 1, 2000, and September 1, 2011, were included. Patients with a synchronous diagnosis of a myeloproliferative or a myelodysplastic disorder or those who developed the myelodysplastic disorder within 15 months of first chemotherapy exposure were excluded. The date of diagnosis for the purpose of this abstract was based on first exposure to chemotherapy and not first detection of a monoclonal protein or smoldering multiple myeloma. All patients had to have morphologic verification in the bone marrow of dysplastic change consistent with therapy-related myelodysplasia.
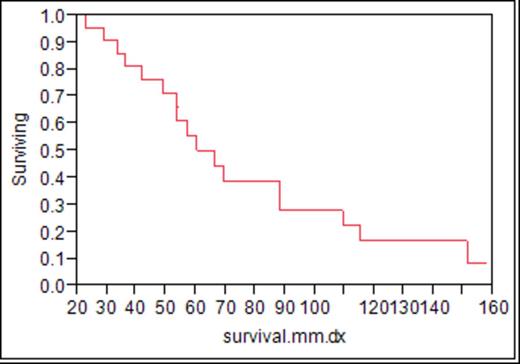
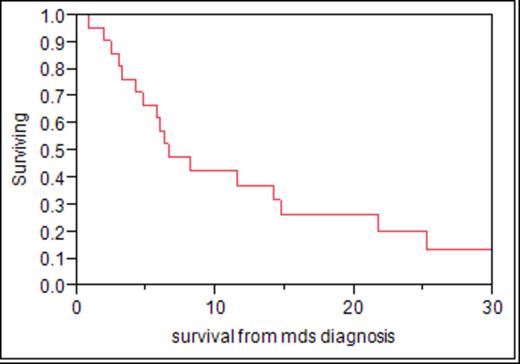
Of 3111 patients who had a myeloma diagnosis and first treatment after January 1, 2000, through September 1, 2011, 22 patients were identified to fulfill the criteria of myelodysplasia. There were 13 men and 9 women with a median age of 67 (46 to 82). At the time of this report, 17 have died. Prior to the development of myelodysplasia, exposure to an alkylating agent, lenalidomide, or an auto stem cell transplant was seen in 13, 15, and 10, respectively. Only one patient had been exposed to lenalidomide alone. Eighteen patients were classified as myelodysplasia. Four had acute non-lymphocytic leukemia. Metaphase cytogenetics was available in 19 and only one was normal. Twelve were hypodiploid. Trisomy 8 or abnormalities of chromosome 5 and 7 were present in 13. Seven patients received a hypomethylating agent with no clear evidence of response in any. Four patients had an allotransplant to manage the MDS/AML and only one survives 487 days after a matched-related donor allogeneic transplant. The median time from initial therapy of multiple myeloma to recognition of MDS or AML was 50.9 months (range: 15.2-146.5 months) fig 1. The median overall survival of the 22 patients is 6.5 months. Fig 2 Six of the 22 had active myeloma at the diagnosis of MDS/AML.
Myelodysplasia during the era of novel agents is a devastating complication of therapy with a very short survival and low response rate. As a time-dependent complication, the overall risk cannot be accurately estimated but has occurred in <1% of our patient population to date. Hypomethylating agents were ineffective in the seven that received it. One of four allotransplant patients remains alive. Ongoing surveillance for MDS/AML in this population is warranted and more effective therapies are needed.
Kumar:Onyx: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal