Abstract
In relapsed or refractory acute myeloid leukemia (AML) long-term disease-free survival may only be achieved with allogeneic stem cell transplantation (HSCT). However, only about 40% of patients (pts) with relapsed AML receive HSCT. A number of factors contribute to this low rate, among them, a moderate activity of currently available salvage regimens and accumulating toxicity of chemotherapy. Clofarabine is considered to have a favorable risk-benefit ratio in this indication and has been successfully used in conditioning regimens. Our goal was to study the safety and efficacy of a clofarabine salvage therapy as a bridge to HSCT. Here, we report the results of the BRIDGE trial (NCT 01295307), a phase II, multicenter, intent-to-transplant study.
Between March 2011 and May 2013, 84 pts with relapsed or refractory AML older than 40 years were enrolled. Pts were scheduled for at least one cycle of induction therapy with CLARA (clofarabine 30 mg/m2 and cytarabine 1 g/m2 days 1-5). Pts with a donor received HSCT in aplasia after first CLARA. In case of a prolonged donor search HSCT was performed as soon as possible. The conditioning regimen consisted of clofarabine 30 mg/m2 day -6 to -3 and melphalan 140 mg/m2 on day -2. In pts with partially matched unrelated donors ATG (Genzyme) at a cumulative dose of 4.5 mg/kg was recommended. GvHD prophylaxis consisted of CsA and mycophenolate mofetil.
Median age was 61 years (range 40 – 75). Forty-four pts suffered from relapsed AML and 40 pts had refractory disease. According to the current ELN risk stratification 17% of pts were classified as favorable risk, 35% as interm. I, 17% as interm. II and 20% as adverse risk. Complex and monosomal karyotypes were present in only 12% and 10% of pts, respectively. FLT3, NPM1 and CEPBA mutations were found in 16%, 24%, and 4% of the pts. The mean value of the HCT-CI score was 1.6 (range 0 - 7) at the time of study enrollment and 2.3 (range 0 - 7) at the time of conditioning.
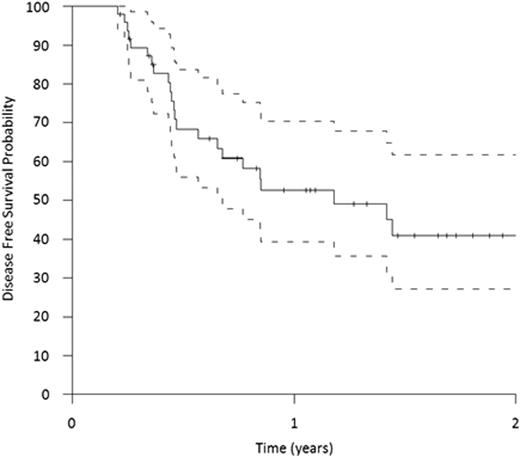
The overall response rate assessed at day 15 after start of CLARA was 80% (46% good response defined as less than 10% blast in the bone marrow (BM) and 33% moderate response with at least a marked reduction in BM blasts or BM cellularity and absence of blast in the peripheral blood). Seventeen pts did not respond to CLARA and were subsequently treated off study. Due to early death, three pts were not evaluable for treatment response. Overall, 66% of the pts received HSCT within the trial. Donors were HLA-identical siblings in eight pts (14%), HLA-compatible unrelated donors in 30 pts (55%) and unrelated donors with one mismatch in 17 pts (31%). Treatment success defined as complete remission, CR with incomplete recovery or >95% BM donor chimerism and an absolute neutrophil count >0.5 /nL on day 35 after HSCT was achieved in 62% of the pts. Disease-free survival (DFS) is shown in Figure 1. With a median follow up of 16 months the OS for all enrolled patients at one year is 51% (95% CI, 39% to 63%).
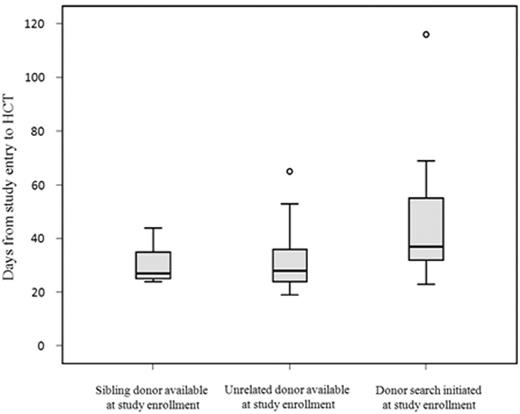
At the time of enrollment, 14% had a related donor and 33% had an unrelated donor. In 46% of the pts donor search was initiated at the time of enrollment. For 7% of pts donor search was not successful. Time from study entry to HSCT was remarkably low with a median of 33 days (range 19 – 116 days). Of note, time interval did not differ between related and unrelated donors (Figure 2).
Time to HSCT according to donor status at the time of study enrollment
Day 30 and day 100 mortality, which covered salvage therapy and HSCT, was 9% and 27%, respectively. Six out of seven pts who died within the first 30 days hat refractory AML and thus entered the trial already with a history of long-lasting neutropenia. Liver toxicity was the most frequent adverse event. Fifty percent of the pts had transiently elevated liver enzymes CTCAE grade III considered to be related to clofarabine. Twenty-one patients developed CTCAE grade III – IV sepsis throughout the study treatment. GvHD grade II – IV and III-IV until day 100 after HSCT occurred in 36% and 21% of the pts, respectively.
This intent-to transplant study allows for a realistic estimate for the outcome of elderly pts with relapsed or refractory AML. We demonstrate a high rate of leukemia-control by CLARA. Fast unrelated donor search and work up and conditioning with clofarabine and melphalan in aplasia allowed for a high rate of successful HSCTs. While the long-term results require longer follow-up the overall results are promising.
Middeke:Genzyme: Speakers Bureau. Schetelig:Genzyme: Research Funding. Off Label Use: Clofarabine, not approved for AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal