Abstract
The diagnosis of classical Hodgkin lymphoma is often difficult to establish due to the rarity of the neoplastic component and the necessity to perform immunostains on serial sections. We have developed a fluorescent multiplexed methodology in formalin-fixed, paraffin-embedded sections which enables assessment of multiple antigens on a single tissue section and allows evaluation of specific cells within specific fields (MultiOmyxTM). In this clinical application, we assessed CD30-positive cells with eight additional antibodies on the same piece of tissue as an aid to the diagnosis of classical Hodgkin lymphoma.
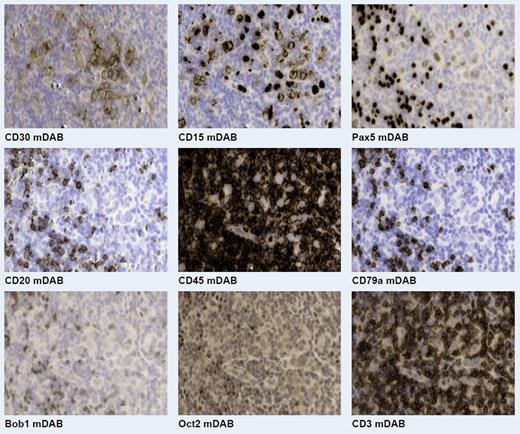
One formalin-fixed tissue section from each of the 56 cases was probed with fluorescently conjugated antibodies against the following nine biomarkers: CD30, CD15, CD45, Pax5, CD20, CD79a, OCT2, Bob1, and CD3 (Fig. 1). An initial 10x whole slide fluorescent image of CD30 was acquired and presented to the pathologist who, based on this staining, selected regions of interest for higher magnification (40x) imaging of the CD30 and the other antibodies to facilitate assessment. The fluorescent images acquired were processed for interpretation using an in-house developed viewing tool. The pathologist was able to view each biomarker as a standard grayscale, monochromatic image, an overlay of two or more biomarkers, or as an algorithmically generated molecular diaminobenzidine (DAB) image resembling a bright field approach.
Representative virtual brightfield images of the nine biomarkers from the same field of view in a Hodgkin Lymphoma sample.
Representative virtual brightfield images of the nine biomarkers from the same field of view in a Hodgkin Lymphoma sample.
The staining characteristics were studied in a series of 23 cases. Individual antibody specificity was found to be 100% in all but one marker assessed; CD30 was found to be discordant in 1 of the 23 samples. Inter- and intraday concordance was 100%. Fifty-six unique cases were studied for diagnostic concordance. We compared historical diagnosis using immunohistochemistry to the MultiOmyxTM diagnosis, studying blinded cases of classical Hodgkin lymphoma and other differential diagnosis entities. Additionally, we included nodular lymphocyte predominance Hodgkin lymphoma, T-cell rich B-cell lymphoma, peripheral T-cell lymphoma, including anaplastic large cell lymphoma, and reactive immunoblastic proliferations. Fifty-four of the 56 cases showed complete concordance. One case was diagnosed as equivocal on initial historical diagnosis, but subsequent rebiopsy diagnosis showed concordance with the MultiOmyxTM diagnosis derived from the initial specimen. One other case was discordant with the historical diagnosis (case was studied five independent times by MultiOmyxTM, all with same diagnosis), and retrospective analysis of the case raised doubt as to the validity of the historical diagnosis.
MultiOmyxTM single slide assay has similar staining characteristics and is at least equivalent to standard immunohistochemical stains. It allows for better correlation of results between stains in a given case, particularly in cases with rare Hodgkin cells, since it allows direct comparison of stains within the same field of view and on the same cells. In addition, MultiOmyxTM may be advantageous in small samples, in which full immunohistochemical profiles may not be possible. MultiOmyxTM allowed improved assessment of Hodgkin cells for antigens expressed on other cell types (e.g., B-cell antigens on reactive immunoblasts, or CD15 on reactive histiocytes), as well as antigens expressed on directly adjacent cells (e.g., CD45 and CD3). This novel methodology is practical for routine diagnosis, and will likely be an aid to the improved diagnosis of Hodgkin lymphoma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal