Abstract
Prior studies have demonstrated weight gain among recipients of chemotherapy for various solid tumors, though there is little evidence describing weight changes during and after treatment in patients with non-Hodgkin lymphoma (NHL). Weight gain during and after treatment can contribute to an increased risk of chronic conditions including: diabetes, coronary disease, and hypertension. This is important for long-term health in patients with diseases that are potentially curable or associated with long-disease free intervals, such as diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL). We investigated the magnitude of weight change during and after treatment in a cohort of DLBCL and FL patients.
DLBCL and FL patients diagnosed between 1998 and 2008 and treated with combination chemotherapy +/- rituximab, were identified in the Veterans Health Administration database. Data on weight at time of first treatment (baseline) and at all recorded weight measurements up to 5 years after treatment initiation was obtained. Additional data included: height, age, race, co-morbidities, date of diagnosis, disease stage, LDH at diagnosis, B-symptoms, and treatment details (drugs, dates, and dosages). Only patients treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) +/- rituximab or CVP (cyclophosphamide, vincristine, and prednisone) +/- rituximab were included in the study cohort. Patients were categorized as gaining or losing 0-2.5%, 2.5-5%, 5-7.5%, 7.5-10%, or >10% of baseline weight. Weight change during treatment was calculated utilizing baseline and weight measurement at or near 3 months following treatment initiation. B-spline modeling was applied to further evaluate trends in weight change over time within the cohort.
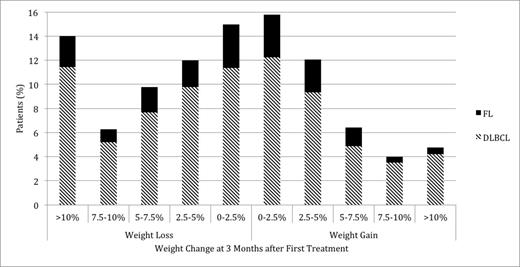
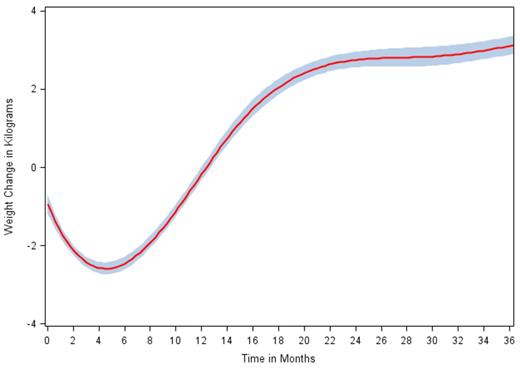
2,159 patients met inclusion criteria. Mean age at diagnosis was 63.1 years, 96.6% of patients were men, and 59.5% of patients had stage III/IV disease. Mean Charlson co-morbidity score was 2.2. B-symptoms were noted at diagnosis in 48.4% of patients and LDH was elevated in 47.2% of patients. Mean and median weight change at 3 months after treatment initiation were -1.9 kg and -1.1 kg respectively, or -2.1% and -1.3% of baseline weight. Figure 1 illustrates the distribution of weight change during treatment within the cohort. In B-spline analysis, weight loss was maximal at 4.14 months after first treatment, with subsequent weight gain until reaching a plateau at 22 months (Figure 2). Among patients with 24 months or more of follow-up data, 45.7% had gained weight at 3 months and 60.5% had gained weight at 24 months. Only 4.3% of patients had gained >10% of baseline weight at 3 months, while 23.0% had gained >10% at 24 months. Similarly, 15.4% of patients had gained >5% of baseline weight at 3 months, while 38.5% had gained >5% at 24 months. Of the patients that gained weight at 3 months, 9.3% had gained >10% of baseline weight, while of the patients that gained weight at 24 months, 38.0% had gained >10%. Patients who gained weight in the first 3 months after treatment initiation were more likely to gain >10% baseline weight at 24 months compared to those who lost weight in the first 3 months.
Distribution of Weight Change at 3 Months after First Treatment among NHL Patients
Distribution of Weight Change at 3 Months after First Treatment among NHL Patients
Penalized B-spline Curve for Weight Change over Time among NHL Patients
These results suggest that in contrast to other malignancies, most patients with NHL who are treated with multi-agent chemotherapy actually experience weight loss rather than weight gain over the course of treatment. Despite this initial trend, a majority of these patients undergo significant weight gain after the conclusion of therapy and in subsequent months. This poses a long-term health risk, particularly to patients who achieve a complete remission and long-term disease control. The timing of greatest weight gain in the 6-18 months after treatment initiation suggests an opportune time for initiation of a diet or exercise intervention to reduce weight gain shortly after the end of treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal