Abstract
Thymopoiesis is a complex process dependent on precise signals from the supporting thymic stromal microenvironment that orchestrates the progression of precursor T cells through well-defined maturation stages. It is well documented that the decline in thymic size and function with age is in part correlated with an increase in sex steroids. This age-related decline in function can be detrimental to the recovery of the thymus in patients receiving radio or chemo-therapy with hematopoietic stem cell transplantation (HSCT). Delayed immune reconstitution, especially in the T cell lineage, is associated with an increased risk of opportunistic infections and malignant relapses. Therefore strategies to enhance thymic reconstitution has the potential to decrease the period of T cell lymphopenia and increase overall clinical outcome.
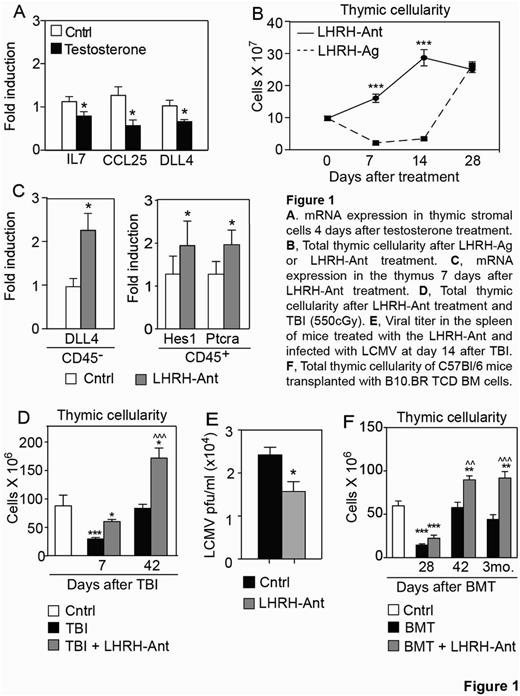
In the process of evaluating the effects of sex steroids in the decline of the thymic function, we found a decrease in the expression of the key thymopoietic factors IL-7, CCL25 and Delta-like 4 (DLL4) by thymic stromal cells after testosterone treatment (Figure 1A). We then addressed if these transcriptional changes were the result of a direct regulation by the androgen receptor (AR). Using a computational approach, and subsequently confirmed by ChIP studies, we found that AR directly bound and negatively regulated the promoter of DLL4, a critical gene involved in T cell commitment and differentiation. We and others have previously shown that sex steroid ablation (SSA) can regenerate young and aged immune system by promoting bone marrow and thymic lymphopoiesis and promoting recovery from autologous and allogeneic HSCT. However the mechanisms underlying the sex steroid-mediate thymic involution and its regeneration after SSA are poorly understood. Moreover, one of the main drawbacks to standard clinical methods of sex steroid ablation using luteinizing hormone releasing hormone (LHRH) agonists (LHRH-Ag) is the initial surge in sex steroids they cause. To address this, we employed a novel class of LHRH-antagonists (LHRH-Ant) that rapidly block the secretion of sex steroids without causing their initial surge that can be even more detrimental to thymopoiesis. Mice treated with LHRH-Ant showed a significantly faster increase in thymic cellularity compared with LHRH-Ag treated mice (Figure 1B). Given the negative regulation of DLL4 by the AR, we hypothesized that DLL4 expression would conversely increase after SSA in vivo. Indeed, we found a significant increase in DLL4 expression after SSA and also an increase in genes downstream of DLL4, such as Ptcra, Hes1 and Cd25 (Figure 1C). We next evaluated if treatment with the LHRH-Ant would provide a faster immune recovery after injury to the immune system. We found that mice treated with LHRH-Ant showed a faster thymic regeneration after total body irradiation (TBI) compared to the control irradiated mice (Figure 1D) and enhanced viral clearance (Figure 1E). Finally, we also found that LHRH-Ant enhanced thymic and peripheral reconstitution up to 3 months after allo-HSCT (Figure 1F).
In conclusion, we found that down-regulation of DLL4 may represent one of the mechanisms underlying the effects of sex steroids on thymic function. We demonstrate that SSA with a novel LHRH-Ant increases DLL4 expression and enhances thymic and peripheral T cell recovery and function after immune injury. These findings suggest that the employment of a LHRH-Ant, which is already in clinical use for prostate cancer patients, represents a novel therapeutic strategy to enhance immune recovery and function in immunocompromised patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal