Abstract
Allogeneic hematopoietic stem cell transplantation (allo-SCT) has become a therapeutic option for patients (pts) with high risk CLL. The major cause of allo-SCT failure is relapse or disease progression. No standard treatment is available for pts who failed allo-SCT and post-SCT immune manipulation, and the prognosis of these pts is largely unknown.
We performed a retrospective intention-to-treat analysis of pts with CLL who failed allo-SCT and post-SCT immune manipulation between 1998 and 2012 and were referred to the Leukemia Department at MD Anderson Cancer Center for further treatment.
Seventy-three pts with CLL (n=40, 55%) or Richter's transformation (RT, n=33, 45%) who failed or progressed after allo-SCT were referred for further management at a median of 7 months (range 0-85 months) after allo-SCT. Most pts (n=63, 86%) had received non-myeloablative conditioning and 32 (44%) received post allo-SCT donor lymphocyte infusion (DLI). Six patients (8%) had early (4 pts) or late (2 pts) graft failure. Fifty four (74%) pts were male and the median age at time of failure/progression was 59 yrs (range 32–73 yrs).
Of the 73 pts, 68 (93%) received salvage therapy, 1 pt died before treatment was initiated, and 4 pts were considering treatment options at time of last follow-up. A median of 2 treatment regimens (range 0 - 8) were administered between time of post-SCT progression and last follow-up. The most common salvage treatment regimens were chemo-immunotherapy with rituximab (R): R-HyperCVAD (n=24, 33%), OFAR (n=13, 18%), FCR, FBR, PCR, BR (n=24, 33%), immune-modulators thalidomide or lenalidomide (n=21, 29%), alemtuzumab with or without chemotherapy (n=12, 16%), and Bruton's Tyrosine Kinase (BTK) inhibitor (n=5, 7%).
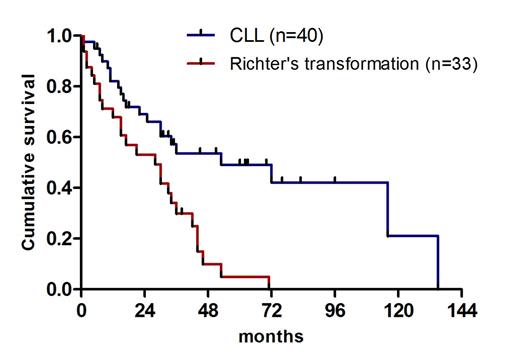
At time of last follow up, 26 pts (36%) were alive. The median overall survival (OS) from time of progression after allo-SCT was 33 months (95% CI, 27– 39) and 60% of the pts were alive 2 years from time of progression. Median OS after allo-SCT failure/progression was 53 months (95% CI, 9–97) for CLL pts and 28 months (95% CI, 12–44) for pts with RT (Log Rank: P<0.0001) (Figure 1). Only 4 pts achieved CR following first salvage treatment. The OS of these pts was 152, 41, 26+, 38+ months (the latter 2 are still alive). Pts with partial response (PR), no-response (NR) or progression after first salvage treatment had similar OS.
Cumulative survival of pts with CLL and Richter's syndrome from time of disease progression after allogeneic stem cell transplantation.
Cumulative survival of pts with CLL and Richter's syndrome from time of disease progression after allogeneic stem cell transplantation.
To identify the best treatment modality we analyzed the OS from progression and from time of initiation of treatment to last follow-up of all regimens used. We found that patients treated with either FCR, alemtuzumab or combination chemotherapy did not have significant difference in OS. Remarkably, 4 of 5 pts treated with BTK inhibitor were alive at time of last follow-up.
Univariable analyses showed shorter OS for pts with a higher ECOG performance status (PS; P=0.036), low hemoglobin (Hb) (P=0.0001), low albumin (P=0.0001), complex cytogenetic abnormalities at last follow up (P=0.026) and RT (P=0.0001). The multivariable Cox model analyses including age, sex, previous DLI, unfavorable cytogenetic abnormalities before or after allo-SCT, albumin, absolute lymphocyte count and platelet count, identified only Hb (P=0.009, 95% CI; 0.58 - 0.93), ECOG 3 (P=0.036, 95% CI; 1.12 - 30.04) and RT (P=0.002, 95% CI: 1.51 - 6.50) as independently associated with shorter OS.
The median OS of pts with high-risk CLL or RT from time of allo-SCT failure/progression was 33 months. The longest OS was observed in pts who attained CR after first salvage treatment (4/73, 6%). At the time of analysis, the 50% OS of pts treated with BTK inhibitors was not reached, however, no treatment modality appeared to be superior. Future studies of treatment options for pts that progress after allo-SCT are warranted.
Off Label Use: Ibrutinib for CLL therapy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal