Abstract
Fludarabine-based therapies prolong survival of patients (pts) with chronic lymphocytic leukemia (CLL), but deplete T-cells causing immunosuppression which increases the risk of infection and may limit control of minimal residual disease by the immune system. Adoptive immunotherapy using autologous CD3/CD28-costimulated T-cells expanded ex vivo (ACTC) enhances immune reconstitution after fludarabine-based therapy of lymphoma (Schuster Blood abstract 2007).
We conducted a multicenter phase I/II trial of ACTC following fludarabine- or alemtuzumab-based therapy of CLL. Eligibility required IWCLL 2008 indications for treatment of CLL. After leukapheresis, pts received fludarabine- or alemtuzumab-based therapy. Eight - 12 weeks after last immuno-chemotherapy, responding pts (CR, PR) received a single dose of ACTC prepared from autologous T-cells collected before therapy. Primary study endpoints were feasibility, defined as the ability to generate a T-cell dose of 1.0 E+10+/- 20%, and safety, defined as <grade 3 non-hematologic toxicity. Secondary endpoints evaluate immune reconstitution following ACTC infusion. Progression-free survival (PFS) was calculated from the date of ACTC infusion (D0) for patients who were evaluable for studies of immune reconstitution at day +90 following ACTC infusion.
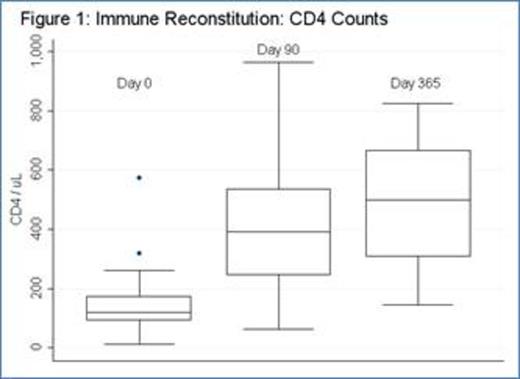
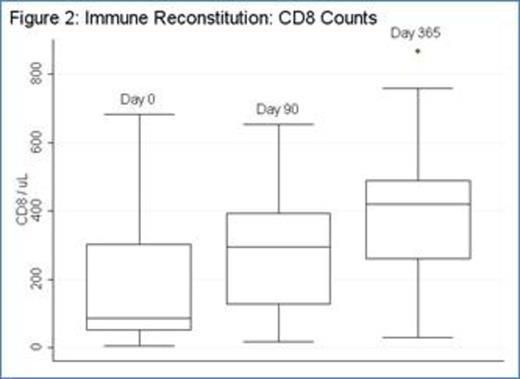
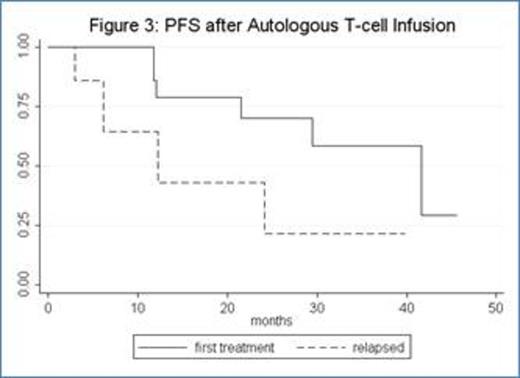
Thirty-four pts with CLL were enrolled (median age: 62). Eighteen patients were previously untreated and 16 patients had relapsed or refractory CLL. Four pts (12%) had del(17p), 11 pts (33%) del(11q), and 3 (9%) both del(17p) and del(11q). For previously untreated patients, 17 received fludarabine-cyclophosphamide-rituximab (FCR) and 1 pt received fludarabine-rituximab (FR); for relapsed patients, 10 received FCR, 3 FCR-bevacizumab, 2 alemtuzumab, and 1 oxaliplatin-fludarabine-cytarabine-rituximab (OFAR). Ten patients did not receive ACTC infusion for the following reasons: 6 pts (including 1 receiving alemtuzumab) had progressive disease prior to ACTC, 1 pt developed autoimmune hemolyic anemia during FCR, 1 pt developed ITP during FCR, 1 pt developed pure red cell aplasia during FCR, and 1 pt receiving alemtuzumab was removed from study per physician preference. Median ACTC dose was 1.0 E+10 CD3 cells (range: 3.94 E+08 -1.03 E+10). Two pts had progressive disease less than 90 days after ACTC and 1 pt was lost to follow-up. Twenty-one pts (14 CR; 2 CRi; and 5 PR at the time of ACTC infusion) were evaluable for studies of immune reconstitution at ≥90 days after ACTC. After chemotherapy prior to ACTC (D0), median CD4 count was 120 (range: 19-573); 90 days after ACTC (D+90), median CD4 count was 399 (range 62-1818) (p = 0.0003; median fold CD4 increase 2.3, range -0.5–20.9) [figure 1]. Median CD8 count also increased significantly (p = 0.004; median D0 = 86, range: 4-682; D+90 = 267, range: 18-2876; median fold CD8 increase 1.1, range: -0.8-18.4) [figure 2]. The increases in CD4 and CD8 counts between D0 and D+90 were not dependent on ACTC dose. There were no SAEs or grade 3 or higher non-hematologic toxicities related to T-cell infusion. For all 21 patients evaluable for immune reconstitution at D+90, median follow-up is 33 months with median PFS 30 months (not shown). PFS for previously untreated patients is 42 months, whereas PFS for relapsed patients is 12 months (figure 3). There was no correlation between magnitude of increase in post-infusion CD4 and CD8 counts and PFS at 1 year.
For CLL patients, ACTC production is feasible. ACTC infusion is well tolerated and results in a dose-independent acceleration of CD4+ and CD8+ cell recovery after fludarabine-based chemotherapy compared to historical controls. The application of adoptive immunotherapy with ACTC to accelerate immune reconstitution after immunochemotherapy could potentially provide an optimal immunologic milieu for early application of additional immunotherapeutic interventions.
Levine:Novartis: cell and gene therapy IP, cell and gene therapy IP Patents & Royalties. June:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal