Abstract
CLL patients (pts) with del(17p13.1) (17p-) karyotype typically have poor response to therapy and dismal clinical outcomes. We describe the success of treatment of 17p- pts on clinical trials at Ohio State University (OSU) with regimens based on 1 of 2 novel agents [ibrutinib (IB; an oral inhibitor of Bruton tyrosine kinase) or one of the cyclin-dependent kinase inhibitors (CDKi; alvocidib, dinaciclib, or TG02)].
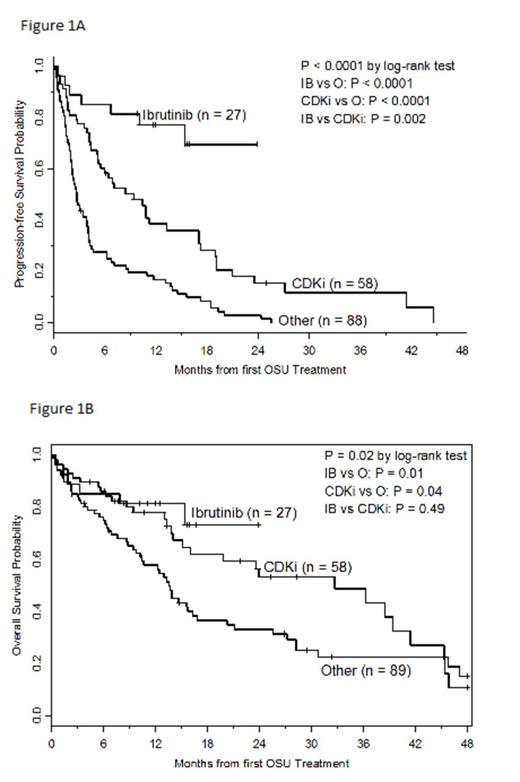
We retrospectively reviewed records of 174 CLL pts with 17p- who received therapy prior to 1st treatment at OSU (OSU Tx1) from 2002-2013. Progression free survival (PFS) was calculated from date of OSU Tx1 until progression/death, censoring pts at date of second treatment prior to progression or last contact if alive and progression free. Overall survival (OS) was calculated from date of OSU Tx1 until date of death. Pts who received transplant or later IB were censored at that time; otherwise pts were censored at last follow-up or 48 mos if still alive. PFS/OS estimates were calculated using the Kaplan-Meier method. Proportional hazard models were fit with treatment group and adjusted for other prognostic variables (p<0.05).
Median time from CLL diagnosis to OSU Tx1 was 5.6 yrs and median number of prior therapies was 3 (range: 1-10). At OSU Tx1, median age was 63 yrs (39-83), 66% were male, and 93% were Caucasian. ECOG performance status (PS) was 0, 1, and 2/3 in 28%, 60%, and 11% of pts, respectively, and 71% had Rai Stage 3/4. In addition to 17p-, 21%, 57%, and 16% of pts harbored 11q-, 13q- and tri(12) aberrations, while 70% had complex karyotype (CK; ≥3 abnormalities including 17p-).
In multivariable analysis, treatment group was significantly associated with achieving at least partial response (p=0.0005) independent of number of prior therapies and presence of 13q-. The odds of response were higher with IB and CDKi compared to O, but not significantly different between IB and CDKi (all pairwise odds ratios (OR): IB vs O=5.66 (95%CI=2.12-15.08); CDKi vs O=3.38 (95%CI=1.57-7.31); IB vs CDKi = 1.67 (95%CI=0.65-4.32)]. Treatment group was significantly associated with PFS (p<0.0001) independent of number of prior therapies, albumin, and CK. The risk of progression/death decreased by 90% and 60%, respectively, with IB or CDKi compared to O (all pairwise hazard ratios (HR): IB vs O=0.10 (95%CI=0.04-0.22); CDKi vs O=0.40 (95%CI=0.27-0.60); IB vs CDKi = 0.24 (95%CI=0.11-0.55)]. Treatment group was significantly associated with OS (p=0.003) independent of number of prior therapies, albumin, CK, ECOG PS, and WBC. The risk of death decreased by 73% and 47%, respectively with IB and CDKi compared to O (all pairwise HR: IB vs O=0.27 (95%CI=0.11-0.66); CDKi vs O=0.53 (95%CI=0.31-0.89); IB vs CDKi=0.52 (95%CI=0.21-1.30)]. Notably, age did not correlate with response, PFS, or OS.
Treatment of 17p CLL pts with IB and CDKi at OSU have demonstrated improved response, PFS, and OS. These novel therapies provide hope for patients with a karyotype historically associated with dismal clinical outcomes.
Jones:Pharmacyclics: Membership on an entity’s Board of Directors or advisory committees, Research Funding. Byrd:Celgene: Consultancy; Johnson and Johnson: Consultancy; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal