Abstract
The mechanism whereby suppression of normal hematopoietic stem cells (HSC) by polycythemia vera (PV) and essential thrombocytopenia (ET) clones occurs remains an enigma, yet PV and ET clones dominate mature myelopoiesis even when increased numbers of circulating clonal cells are normalized by hydroxyurea or other myelosuppressive therapies. Interferon-a (Infa) is the only treatment that not only decreases JAK2V617F allelic burden and ameliorates marrow cytogenetic abnormalities, but may also reverse clonal hematopoiesis (E. Liu, Blood, 2003). The mechanism of this response is not certain, and some data suggest this reversal may be due to: a) increased cycling of normal HSC (see K. King abstract at this ASH mtg), b) possible immune response against the malignant clone, or c) direct suppression of the malignant clone by an as-yet-unknown mechanism. As TNFa is a cytokine known to suppress normal hematopoiesis, we have examined its role in possible reversal to polyclonal hematopoiesis in patients treated in a Phase III trial for PV and ET with either pegylated interferon-α (pegInfa) or hydroxyurea.
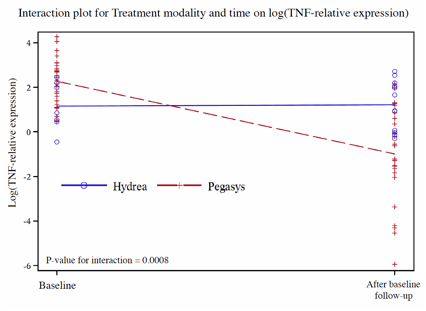
We found that plasma TNFa levels were not markedly elevated in PV and ET patients (range = 1-10 pg/ml, mean = 4 pg/ml, normal = <5 pg/ml) and did not change before or after therapy with pegInfa or hydroxyurea. However, immunohistochemical staining of PV and ET marrows showed a marked increase in TNFa protein expression in PV and ET marrow cells compared to normal controls. We found that TNFa mRNA, measured by QT-PCR, of early and late myeloid progenitors isolated from marrow was mainly confined to early hematopoietic precursors. Furthermore, sorted PV & ET CD34+ cells had increased TNFa transcripts when compared to normal HSC cells (17 fold, p>0.0005). Patients who responded to therapy with pegInfa had a greater decrease in TNFa transcripts in CD34+ cells compared to patients treated with hydroxyurea (p value =0.0008) (see Figure). In pegInfa-treated patients, the decrement in TNFa mRNA corresponded to the decline in JAK2V617F level (Spearman's Rho = 0.4254; p-value = 0.0097). Additionally, 2 female PV patients who converted from clonal to polyclonal hematopoiesis (detected by X-chromosome allelic usage ratio) also had marked decline of CD34+ TNFa mRNA. Furthermore, 1 PV patient, who had a dramatic decline of JAK2V617F and TNFa mRNA on pegInfa, which was stopped because of side-effects and then switched to hydroxyurea, reverted to increased CD34+ TNFa mRNA and an increased JAK2V617F allelic burden. Then, because of an incomplete response to hydroxyurea, this patient was started on low-dose pegInfa and had marked corresponding changes of both JAK2V617F and TNFa. In 2 pegInfa-treated PV and 1 untreated PV patients, 9 JAK2V617F homozygous BFU-E, 35 JAK2V617F heterozygous BFU-E, and 64 wild-type JAK2 BFU-E colonies were analyzed for TNFa transcripts. Unexpectedly, at the time of harvest of BFU-E colonies (14 days after culture, cells were largely late normoblasts), the JAK2V617F-positive colonies had undetectable TNFa transcripts, unlike wild-type JAK2 in PV BFU-E and BFU-E colonies from normal healthy controls.
These data indicate that intracellular and possibly paracrine TNFa is strongly associated with the suppression of normal hematopoiesis in PV and ET. We demonstrated that this increase in HSC TNFa is markedly reduced in those patients successfully treated with pegInfa, and this decrease is not seen when increased numbers of circulating clonal cells are normalized by hydroxyurea. These data provide a foundation for the addition of TNFa inhibitors as possible combination therapies for PV & ET.
This work was supported by 1P01CA108671-O1A2 (NCI) Myeloproliferative Disorders (MPD) Consortium (PI Ron Hoffman) project#1 (PI Prchal) and Leukemia&Lymphoma Society.
First and second authors contributed equally
Swierczek:University of Utah: No financial compensation, No financial compensation Patents & Royalties. Prchal:University of Utah : No financial compensation, No financial compensation Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal