Abstract
Bcr-Abl1 is necessary and sufficient to cause chronic myeloid leukemia (CML) and as such CML cells are dependent on Bcr-Abl signalling for survival. Targeting CML cells with tyrosine kinase inhibitors (TKIs) commits cells to apoptotic cell death. Bcr-Abl constitutively activates STAT5, however the role of JAK-2 in the activation of STAT5 by Bcr-Abl is controversial. Recent studies of transient Bcr-Abl inhibition indicate that residual low levels of TKI are sufficient to maintain STAT5 inhibition in the absence of sustained Bcr-Abl inhibition. Therefore STAT5 is a highly sensitive measure of kinase activity. We hypothesized that sustained blockade of STAT5 is essential for the commitment of CML cells to apoptosis following inhibition of Bcr-Abl by TKIs.
To determine the role of STAT5 and JAK inhibition in the commitment of CML cells to apoptosis.
Factors required for CML cell death were examined in the setting of transient inhibition of Bcr-Abl by TKIs. Induction of apoptosis was assessed by Annexin V/7AAD and the clonogenic potential of CML progenitors assessed by CFU-GM assay. Bcr-Abl and apoptotic signaling pathways were interrogated by western blotting and flow cytometry. Dasatinib was used at 100 nM for potent inhibition of Bcr-Abl. Short term refers to 30 min exposure. Standard washout refers to 3 consecutive washes following potent TKI treatment. Optimal washout refers to 3 washes with 1 h equilibration at 37°C in drug free media between washes.
In BCR-ABL+ cell lines short term, potent dasatinib exposure followed by optimal washout resulted in reactivation of Bcr-Abl and STAT5, inhibition of apoptosis (83% viable, n=3) and maintenance of colony formation in CML progenitors (CFU-GM: 85% of untreated n=3).
Plasma concentrations of dasatinib vary between patients, however peak plasma levels occur up to 6 h after dosing and dasatinib remains available for up to 24 h. CML cell lines and CP-CML CD34+ progenitors were exposed to 100 nM dasatinib for 0.5-8 h before optimal washout. Cell death was achieved if TKI exposure by at least 4 h, with maximal cell death (15% viable, n=3, p=0.008) and reduction of colonies (30.1% of control, p=0.002) achieved after 8 h exposure. Comparison of 30 min and 8 h exposures to 100 nM dasatinib followed by optimal washout was performed to assess the critical signalling components required to induce apoptosis. Reactivation of Bcr-Abl, STAT5 and Erk occurred upon washout following both the 30 min and 8 h exposures, however the 8 h exposure resulted in the inhibition of STAT5 and loss of expression of STAT5 targets Mcl-1 and Bcl-xl, but not Bcl-2. In CP-CML CD34+ cells, prolonged inhibition of STAT5 was observed after 4 h exposure, following optimal washout, highlighting loss of STAT5 activity as potentially critical to irreversible induction of cell death.
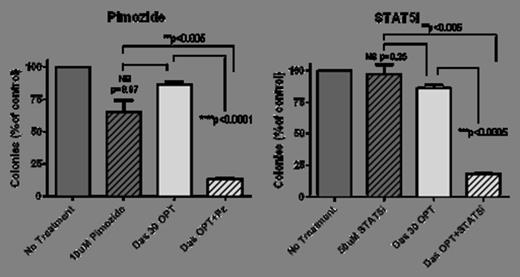
Continuous inhibition of STAT5 alone with pimozide (Pz) or the specific inhibitor N’-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydrazide (herein referred to as STAT5i) led to minimal apoptosis (73% and 75% viable, respectively, n=3) when used alone. However, when combined with 30 min exposure to dasatinib (100 nM) STAT5 inhibition proved lethal in a proportion of cells despite optimal washout (57% viable +Pz and 59% +STAT5i). The clonogenic potential CML progenitors was also significantly reduced (12%, p=0.002 and 18% CFU, p=0.003) (Figure 1).
Combination of STAT5 inhibition with 30 min dasatinib exposure (100 nM) reduces the colony forming ability of CP-CML CD34+ cells. (OPT = optimal wash)
Combination of STAT5 inhibition with 30 min dasatinib exposure (100 nM) reduces the colony forming ability of CP-CML CD34+ cells. (OPT = optimal wash)
The JAK1/2 kinase inhibitor ruxolitinib was used to assess the involvement of JAK1/2 in Bcr-Abl-dependent activation of STAT5. Similar to the observations with STAT5 inhibition, ruxolitinib had minimal effect on cell death as a sole agent (74% viable). However, in contrast to our observations with STAT5 inhibition, the addition of ruxolitinib to 30 min 100 nM dasatinib exposure did not induce additional cell death (70% viable, p=0.41, n=3).
STAT5 is a critical component of the time-dependent sensitivity of CML cells to TKI treatment in a Bcr-Abl-dependent, but JAK-independent manner. In contrast to previous studies describing JAK2 as a promising secondary target for the enhancement of TKI treatment of CML, we demonstrate that inhibition of STAT5 in conjunction with standard TKI therapy is a promising therapeutic strategy for the treatment of CML.
Nievergall:CSL: Research Funding. White:Novartis: Research Funding; BMS: Research Funding, Speakers Bureau; Ariad: Research Funding; CSL: Research Funding. Hughes:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Ariad: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; CSL: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal