Abstract
Although almost 30% of adult acute lymphoblastic leukemia (ALL) cases occur in patients (pts) 60 years and older, available data on ALL in older pts are scarce. Older ALL pts are often excluded from clinical trials that apply the same intense treatment regimen developed for younger pts, and the outcome of unselected older ALL pts receiving either intensive or minimal treatment remains largely unknown. We therefore conducted a single-institution retrospective study to examine the treatment pattern and outcome of adult ALL pts aged 60 and older and to provide background information to guide the design of future prospective interventional studies.
Pts aged 60 and older with a diagnosis of ALL who were referred to Memorial Sloan-Kettering Cancer Center between 1986 and 2010 were identified. Individual medical and pharmacy records were reviewed to obtain data on age, gender, diagnosis, treatment, treatment-related complications, response rates, survival, and medical comorbidities including previous malignancies. The induction treatment approaches were divided into two categories: 1) minimal, defined as consisting of treatment with vincristine, corticosteroid, with or without a third drug, and 2) intensive, defined as consisting of >3 drug-based combination chemotherapies.
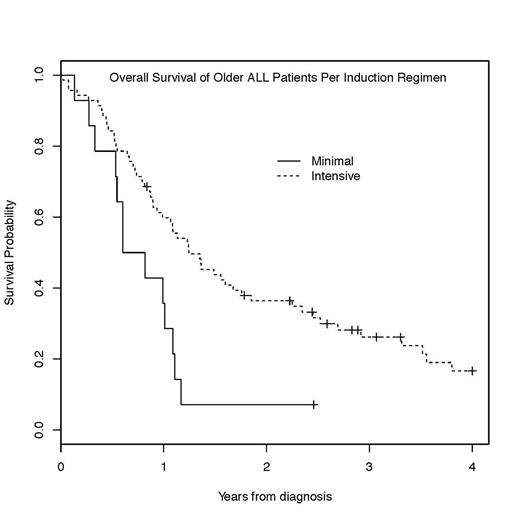
A total of 90 pts aged 60 and older at the time of ALL diagnosis were identified. The median age was 66 years (range, 60 – 83). 19 pts (21%) had a previous diagnosis of another malignancy, for which 9 pts received systemic chemotherapy or radiation. 12 pts (13%) had T-ALL, and 78 pts (87%) had B-ALL, including 28 pts with Philadelphia chromosome (Ph)-positive B-ALL (31%). All 90 pts received induction chemotherapy; 76 pts (84%) and 14 pts (16%) received the intensive and minimal induction regimen, respectively. Of 28 pts with Ph+ B-ALL, 24 pts (86%) received TKI (imatinib or dasatinib) during induction. The median age of pts in the intensive cohort was significantly younger (p < 0.001), 65.5 years (range, 60 – 76) vs. 77.5 years (range, 62 – 83). The overall rate of complete response (CR) was 83%, and no significant difference in CR rates was observed between the intensive and minimal induction treatment groups (84% vs. 77%, p=0.69). Treatment-related complications were more common in the intensive induction group, including infections (85% vs. 50%), hemorrhage/DVT/PE (31% vs. 14%), and physical deconditioning requiring rehab stay (24% vs. 14%). 5 pts died during induction, all due to infectious complications. Of 73 pts who achieved CR following induction, 62 pts (85%) proceeded to post-remission therapy including 13 transplants (21%). Post-remission therapy was delayed due to induction treatment-related toxicities in 35% and 23% of pts in the intensive and minimal induction cohorts, respectively, and 8 relapses occurred due to delay in treatment. Pts who received the intensive induction had high rates of treatment-related mortality (16%) during the post-remission therapy. The median survival for the entire study population was 13.1 months (95% CI: 10.7-17.9), and a significant difference in survival rates (14.9 vs. 8.5 months) was observed between the intensive and minimal induction cohorts, respectively (p= 0.009, Figure). The major cause of death was either infectious complications or relapsed/refractory ALL. Long-term survivors were scarce, with only 5 pts (5.5%) surviving longer than 5 years.
To the best of our knowledge, this is the largest study evaluating the outcome of unselected older ALL pts outside of clinical trial setting. Our study indicates that regardless of induction approaches and even with minimal chemotherapy, older ALL pts can achieve a high CR rate, comparable to that observed in younger adults. This finding may suggest lesser biological difference between younger and older ALL, in contrast to increasing rates of primary chemotherapy resistance with aging in AML. However, compared to younger ALL adults, older ALL pts experience exceedingly high rates of treatment-related complications, and do not gain sustainable benefit from aggressive post-remission treatment with dismal long-term outcome. Therefore, future prospective trials of frontline therapy in older ALL should focus on developing treatment approach to sustain the remission with novel, less toxic agents, and better define distinct biological risk factors within this age group.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal