Abstract
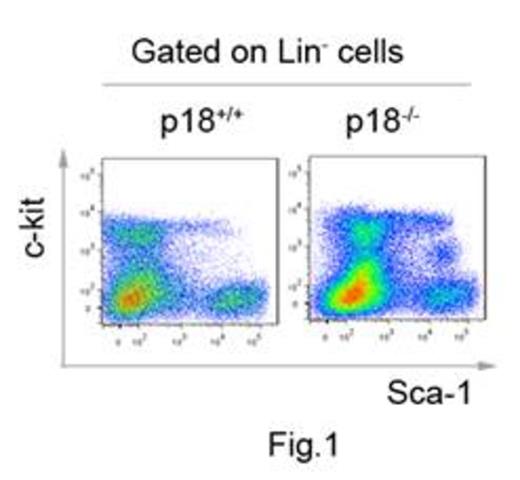
p18INK4C (CDKN2C, p18) is a negative regulator of cell cycle progression at early G1 phase. Deletion of p18 was indicated to be associated with T cell malignancies in mice and B cell malignancies in humans. It is an important indicator for the poor prognosis of patients with multiple myeloma. While the pathological effects of p18 absence can be attributed to the disruption of B cell differentiation or the hyper-response of T cell activation, the potential contribution of hematopoietic stem cell (HSC) to the pathogenesis or defense in the absence of p18 is unknown. This is a logical and important question because p18 is also a known inhibitor for self-renewal of HSC and HSC has been thought to be involved in most if not all the hematopoietic malignancies.
Our ongoing studies are determining the direct contribution of LSKlow cells to the lymphoid malignancies in the absence of p18. Nevertheless, this work reveals an interesting paradigm in which a new stem or progenitor cell population may emerge in the absence of a specific gene and this new stem cell like population due to the genetic abnormality may potentially participate in disease pathogenesis or defense.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal