Abstract
Following intravascular hemolysis, free hemoglobin A is bound to haptoglobin (Hp), internalized by monocytes bearing the hemoglobin scavenger receptor, CD163, and broken down via the heme-oxygenase (HO)-1 enzyme into the cytoprotective metabolites biliverdin and carbon monoxide. This triggers an IL10 positive feedback loop which augments CD163 expression; thereby increasing hemoglobin scavenging capacity and limiting the vascular insult caused by oxidative free heme. During iatrogenic hemolysis caused by cardiopulmonary bypass, we have demonstrated that serum Hp is temporarily consumed while the surface CD163 and HO-1 expression is up-regulated. In homozygous sickle cell disease (HbSS), chronic hemolysis is linked with exhaustion of serum Hp and suppression of CD163 compared to HbAA controls. Our objective was to determine if the extent of Hp depletion correlates with the CD163 suppression and with select clinical features within the Barbadian HbSS population.
Persons with HbSS were referred from private and public hematology clinics and support groups. Exclusion criteria were blood transfusion in the last three months, a sickle crisis in the last month and the use of oral corticosteroids, since these may affect CD163 expression. A history of painful crises, leg ulcers, renal compromise and pulmonary hypertension was ascertained. Urine samples were analyzed for albumin:creatinine ratio and echocardiograms were analyzed for the tricuspid regurgitant jet velocity (TRJV). Whole blood was collected in EDTA and analyzed by two color flow cytometry for the mean fluorescent intensity (MFI) of CD163 expression on gated CD14+ monocytes. Plasma Hp was analyzed by ELISA. The association between Hp concentration and CD163 was analyzed using median regression. The effect of Hp concentration on clinical outcome was explored using logistic regression, adjusting for the effects of age, sex, and total hemoglobin.
Forty-eight persons with HbSS participated. Forty-seven percent of participants had at least one painful crisis in the past year and 28% of participants had a history of chronic leg ulceration. Forty-one persons had urinalysis, of these 44% of persons were diagnosed with renal compromise as defined by a urinary protein/creatinine ratio of >30mg/g; at least 15% had macro-albuminuria (urinary protein/creatinine ratio of >300mg/g). Thirty-two participants had echocardiograms, 19% indicated pulmonary hypertension (pHTN) as defined by a TRJV ≥ 2.5m/s.
The mean (95% CI) Hp concentration was 4 (3.2 to 4.8) [reference range in HbAA 41-165mg/dl]. Linear regression showed that for every 1mg/dl increase in Hp concentration, CD163 MFI increased by 0.17 (95% CI 0.05 – 0.3) p=0.01 (Figure 1).
Association between Hp concentration (mg/dl) and CD163 (MFI). For every 1mg/dl increase in Hp concentration, CD163 MFI increased by 0.17 (95% CI 0.05 – 0.3) p=0.009
Association between Hp concentration (mg/dl) and CD163 (MFI). For every 1mg/dl increase in Hp concentration, CD163 MFI increased by 0.17 (95% CI 0.05 – 0.3) p=0.009
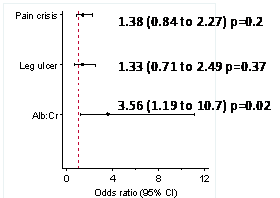
For every 1mg/dl decrease in Hp levels; the odds of reporting a pain crisis in the last year increase by 1.38 (95%CI 0.84 to 2.27) p=0.2; the odds of having renal compromise increase by 3.56 (1.19 to 10.7) p=0.02 and the odds of reporting a history of leg ulcer increase by 1.33 (0.71 to 2.49) p=0.37 (Figure 2).
The effect size of pain crisis history, leg ulcer history and renal status for every 1mg/dl decrease in Hp levels.
The effect size of pain crisis history, leg ulcer history and renal status for every 1mg/dl decrease in Hp levels.
For every 1mg/dl decrease in Hp, the TRJV increases by 0.10 (-0.15 to 0.34) p=0.36.
Depletion of serum Hp correlates with diminished CD163 expression and with increasing severity of clinical features in HbSS. This suggests that the fault in the hemoglobin scavenging apparatus is probably due to chronic depletion of Hp. Replacement of Hp may therefore be a viable therapeutic prospect aimed at restoring the positive feedback loop and conferring vascular protection.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal