Abstract
aHUS is a rare, genetic, life-threatening disease of uncontrolled and chronic complement activation, leading to systemic TMA and severe end-organ damage. Despite plasma exchange/plasma infusion (PE/PI), up to 65% of all pts sustain permanent renal damage, progress to end-stage renal disease, or die within a year of diagnosis. Ecu, a terminal complement inhibitor, is approved for the treatment of aHUS, and was shown to be safe and effective in pediatric pts in a retrospective study. Previous studies have shown that early intervention with Ecu leads to significant, time-dependent improvements in hematologic and renal outcomes. Here, we report safety and efficacy results from the first prospective trial of pediatric pts with aHUS at 26 weeks (wks).
This was an open-label, single-arm, Phase 2 trial of Ecu in pediatric pts with aHUS. Admission criteria included platelet count < the lower limit of normal (LLN) at screening and at baseline (BL), LDH ≥1.5 times the upper limit of normal (ULN) at the start of the current aHUS manifestation, and elevated serum creatinine (Cr) at screening. An identified complement gene mutation was not required. Pts with STEC-HUS (shiga toxin + E. coli) or severe ADAMTS13 deficiency (<5%) were excluded. The primary endpoint was complete TMA response at 26 wks (normalization of platelets and LDH, and ≥25% improvement in Cr from BL on 2 consecutive measurements ≥4 wks apart). Dosing was based on weight cohorts.
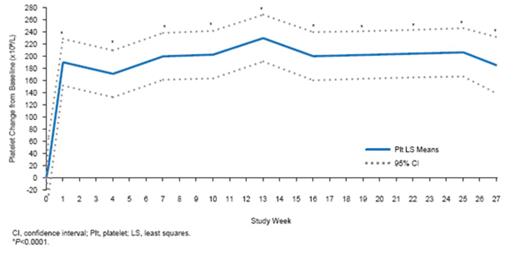
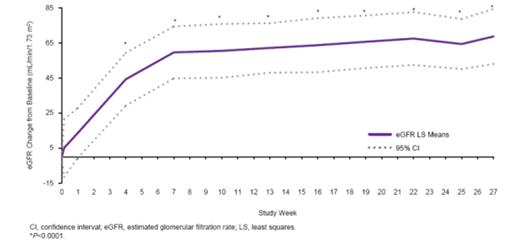
22 pts (1 month to 17 yrs) were enrolled and 19 completed 26 wks. 16 pts (73%) were newly diagnosed, with a median of 6 days from diagnosis to the first dose of ECU. 12 pts (55%) had no PE/PI during the current manifestation. At wk 26, 14 pts (64%) achieved the primary endpoint of complete TMA response (Table). Platelets (Fig 1) and eGFR (Fig 2) increased significantly from BL through the 26-wk study period. 9 of 11 pts (82%) on dialysis at BL discontinued dialysis. 100% of the 11 pts not on dialysis at BL remained off dialysis through wk 26. Mean increase in eGFR through wk 26 was 64 mL/min/1.73m2. All 10 pts on PE/PI at BL discontinued PE/PI. QoL significantly improved. Ecu was safe and well tolerated. 1 pt withdrew due to an SAE (agitation). No pts had meningococcal infection or died.
Figure 1: Platelet count improvement through 26 weeks.
Figure 2: eGFR improvement through 26 weeks.
Table
| Baseline Demographics and Disease Characteristics (N=22) | |
| Age, mean (SD), y | 6.6 (6.1) |
| Female sex, n (%) | 10 (45) |
| Identified complement regulatory protein mutation or auto-antibody, n (%) | 10 (45) |
| Time from aHUS diagnosis until screening (mo), median (range) | 0.56 (0.03–191.3) |
| Duration of current clinical manifestation of aHUS (mo), median (range) | 0.2 (0.0–4.3) |
| Dialysis at baseline, n (%) | 11 (50.0) |
| Prior renal transplant, n (%) | 2 (9) |
| Platelet count <150x109/L, n (%) | 22 (100) |
| LDH >ULN, n (%) | 19 (86) |
| eGFR ≤60 mL/min/1.73m2,n (%) | 18 (82) |
| Efficacy Outcomes at Wk 26 | |
| Complete TMA response*, n (%) | 14 (64) |
| Complete hematologic response†, n (%) | 18 (82) |
| Platelet count normalization‡, n (%) | 21 (95) |
| eGFR improvement from baseline ≥15 mL/min/1.73m2, n (%) | 19 (86) |
| eGFR increase from baseline (mL/min/1.73 m2), mean (95% CI) | 64 (50; 79) P<0.0001 (wk 25) |
| Serum creatinine ≥25% decrease from baseline, n (%) | 16 (73) |
| Baseline Demographics and Disease Characteristics (N=22) | |
| Age, mean (SD), y | 6.6 (6.1) |
| Female sex, n (%) | 10 (45) |
| Identified complement regulatory protein mutation or auto-antibody, n (%) | 10 (45) |
| Time from aHUS diagnosis until screening (mo), median (range) | 0.56 (0.03–191.3) |
| Duration of current clinical manifestation of aHUS (mo), median (range) | 0.2 (0.0–4.3) |
| Dialysis at baseline, n (%) | 11 (50.0) |
| Prior renal transplant, n (%) | 2 (9) |
| Platelet count <150x109/L, n (%) | 22 (100) |
| LDH >ULN, n (%) | 19 (86) |
| eGFR ≤60 mL/min/1.73m2,n (%) | 18 (82) |
| Efficacy Outcomes at Wk 26 | |
| Complete TMA response*, n (%) | 14 (64) |
| Complete hematologic response†, n (%) | 18 (82) |
| Platelet count normalization‡, n (%) | 21 (95) |
| eGFR improvement from baseline ≥15 mL/min/1.73m2, n (%) | 19 (86) |
| eGFR increase from baseline (mL/min/1.73 m2), mean (95% CI) | 64 (50; 79) P<0.0001 (wk 25) |
| Serum creatinine ≥25% decrease from baseline, n (%) | 16 (73) |
Complete TMA response: normalization of platelets and LDH, and ≥25% improvement in Cr from BL on 2 consecutive measurements ≥4 wk apart.
Complete hematologic response: platelet and LDH normalization at ≥2 consecutive measurements ≥4 wk apart.
Platelet count normalization: platelet count ≥150x109/L at ≥2 consecutive measurements ≥4 wk apart.
In this, the first prospective trial of pediatric pts with aHUS, early intervention with Ecu improved hematologic and renal parameters. 82% of pts discontinued dialysis, and no pt initiated dialysis during the 26-wk study. Use of Ecu has been recommended as first-line therapy in children with aHUS. This trial confirms that Ecu inhibits complement-mediated TMA, and is safe and effective as first-line therapy at the approved dose regimen in pediatric pts with aHUS. Treatment in this clinical trial is ongoing.
Greenbaum:Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding. Ardissino:Alexion Pharmaceuticals: Formal Advisory Activities Other, Honoraria. Henning:Alexion Pharmaceuticals: Grant Support Other, Research Funding. Pape:Novartis Pharmaceuticals, Alexion Pharmaceuticals: Grant Support Other, Honoraria, Research Funding, Speakers Bureau. Kar:Alexion Pharmaceuticals: Member of the Int. Advisory Board aHUS Other. Vande Walle:Alexion Pharmaceuticals: Ferring Safety Board, Astellas Safety and Investigator Board for Solifenacin, Mitsubishi Safety Board, Alexion Registry Review Board Other, Research Funding, Speakers Bureau. Ogawa:Alexion Pharmaceuticals: Employment. Bedrosian:Alexion Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal