Abstract
Atypical hemolytic uremic syndrome (aHUS) is a genetic, life-threatening, chronic, and progressive disease of thrombotic microangiopathy (TMA). Plasma exchange/plasma infusion (PE/PI) has been shown to lack efficacy in patients (pts) with aHUS. Despite PE/PI, up to 65% of pts sustain permanent renal damage, progress to end-stage renal disease, or die within 1 year (yr) of diagnosis. Among aHUS pts with long disease duration and CKD receiving chronic PE/PI, significant improvements in hematologic parameters and renal function were achieved in a clinical trial of eculizumab (Ecu). The current analysis was undertaken to gain better insight into the timing of hematologic and renal improvements in a 26-week (wk), Phase 2 trial with a long-term extension.
aHUS pts ≥12 yrs of age with long disease duration and CKD receiving chronic PE/PI were enrolled. This analysis assessed the percentage of pts achieving each of the following outcomes – all for ≥2 consecutive measurements, ≥4 wks apart – at specific time points: Platelet count normalization (≥150x109/L); LDH ≤ULN; serum creatinine (Cr) decrease ≥25%; eGFR increase ≥15 mL/min/1.73 m2; and CKD improvement ≥1 Stage.
20 pts aged ≥12 yrs receiving long-term PE/PI were enrolled and treated with Ecu in a 26-wk, single-arm, Phase 2 trial, and 19 continued in the extension study. The median time (range) from aHUS diagnosis to screening was 48.3 months (0.7–285.8), and the median time from the current manifestation of aHUS to screening was 8.6 months (1.2–45). The median duration of Ecu treatment at the time of the data cut was 114 wks. Mean baseline values were as follows: platelet – 228x109/L; Hb – 10.7 g/L; LDH – 223 U/L; Cr – 287 μmol/L; and eGFR – 30.8 mL/min/1.73 m2. 3 pts had platelet counts <150 x109/L at baseline, and 4 had LDH levels >ULN.
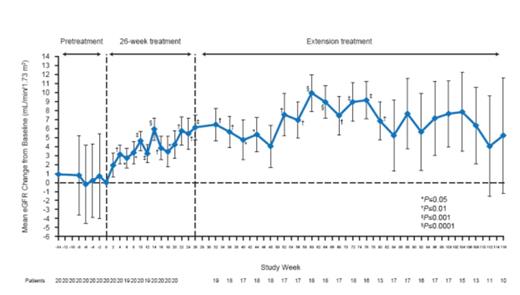
The timing and duration of the criteria-defined hematologic and renal improvements during continued treatment with Ecu are shown in Figure 1. At wk 4, the percentage of pts achieving platelet and LDH normalization was 75% and 50%, respectively (the first assessable time point based on the criteria definition). 90% of pts had platelet count normalization by wk 8, which was sustained with ongoing Ecu treatment for the remainder of the study period. 85% of pts had LDH ≤ULN by wk 8, which increased to 95% by wk 12 (Figure 1). With ongoing Ecu treatment, 10% of pts achieved Cr decrease (≥25%) at wk 14, and 55% by wk 80. eGFR increase (≥15 mL/min/1.73 m2) was first seen at wk 18 (by 5% of pts). This proportion increased to a maximum of 40% at wk 104 with ongoing Ecu treatment. CKD improvement (≥1 Stage) was seen at wk 4 by 5% of pts. This proportion increased to 60% at wk 76 with ongoing Ecu treatment (Figure 1). Significant mean changes from baseline in eGFR were observed as early as wk 4, and were followed by time-dependent improvements through the end of the study (Figure 2). All patients were able to discontinue PE/PI.
Percentage of patients achieving criteria for sustained improvement over time for hematologic and renal parameters*,†,‡
Percentage of patients achieving criteria for sustained improvement over time for hematologic and renal parameters*,†,‡
The use of Ecu in aHUS pts with long disease duration and CKD on long-term PE/PI led to sustained normalization of hematologic values within the first month of treatment, followed by time-dependent improvements in renal function. These data demonstrate that improvement in renal function may be achieved over time in pts with long-standing aHUS and CKD, and underscore the importance of ongoing and consistent treatment with Ecu. The underlying mechanism of the observed renal function improvement over the study period (e.g., normalization of endothelial cell function) warrants further exploration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal