Abstract
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is applied for a wide range of malignant hematologic diseases at a wide range of stages of activity, from durable complete remission to resistant relapse. Reporting transplant outcome by disease category alone, without considering disease activity, limits statistical analysis and interpretation of outcomes across time or institutions. Recently a tool for clustering different combinations of disease and disease stage – the Disease Risk Index (DRI) – was developed and validated to stratify for overall survival (OS), progression free survival (PFS) and cumulative incidence of relapse (CIR)1.
1. To independently validate the DRI's ability to stratify patients for OS, PFS and CIR using data from our centre. 2. To determine if absolute estimates of OS, PFS and CIR derived from the original training cohort can be used to accurately predict OS, PFS and CIR for an independent, contemporaneous cohort. 3. To determine approximate thresholds of sample size and follow-up duration below which the DRI may fail to successfully stratify patients for OS, PFS and CIR.
From our institutional transplant database, we extracted data from 466 patients undergoing alloHSCT for hematologic malignancy at our institution between the years 2001 and 2011, with median survivor follow-up of 55.2 months (range 5.0-139.0) to validate and further explore the utility of the DRI. Data for time-to-event outcomes was locked for analysis on 14 February 2013.
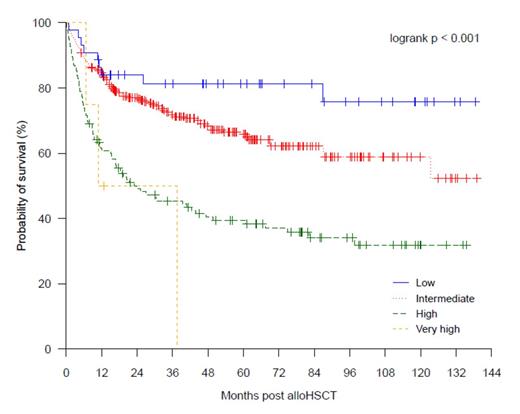
We identified that similar to the published findings, the DRI was able to significantly stratify for OS (at 4 years, OS for low DRI 81% [95% CI 70-94%]; intermediate DRI 68% [63-74%]; high DRI 41%, [32-51%]; very high DRI 0%; P < .001; see Figure 1), PFS (at 4 years, PFS for low DRI 72% [59-87%]; intermediate DRI 61% [55-67%]; high DRI 32% [24-42%]; very high DRI 0%; P < .001), and CIR (at 4 years, CIR for low DRI 14% [3-25%]; intermediate DRI 27% [21-32%]; high DRI 48% [39-57%]; not calculable for very high DRI; P < .001). The DRI was not predictive of non-relapse mortality (P = .379). The DRI retained its prognostic power when applied to subgroups of patients who received either myeloablative or non-ablative conditioning; non-T cell depleted transplants; and sibling donor transplants only. When compared with the original published training cohort, survival and relapse outcomes from a contemporaneous cohort (n = 324, alloHSCT between the years 2001 and 2008) from our institution were superior to those of the Boston cohort for low, intermediate and high DRI groups (4 year OS: low DRI 88% [95% CI 77-100%] vs 64% [56-70%]; intermediate DRI 68% [62-75%] vs 46% [42-50%]; high DRI 42% [33-53%] vs 26% [21-31%]; 4 year CIR: low DRI 12% [6-24%] vs 19% [13-24%]; intermediate DRI 26% [20-32%] vs 36% [33-40%]; high DRI 47% [37-57%] vs 55% [50-60%]). These findings underscore the importance of calibration with local data before using the DRI for predicting absolute rates of survival and relapse in individual institutions. To further explore if the DRI retained its power in smaller cohorts, we tested the DRI in smaller subsets of patients selected randomly from our data. We found the DRI successfully stratified a cohort of 100 patients (median survivor follow-up 51.1 months, range 8.5-139.0) for OS (P = .010), PFS (P = .016) and CIR (P = .027), but failed to stratify a cohort of 50 patients (median survivor follow-up 46.2 months, range 10.3-135.5) for survival (P = .385 for OS, P = .167 for PFS, P = .026 for CIR). Likewise, under simulated conditions of shorter follow-up, the DRI successfully stratified a cohort (n = 322) with median surviving follow-up of 40.6 months for OS and PFS, but failed to stratify a cohort (n = 242) with median surviving follow-up of 33.1 months.
Overall survival according to Disease Risk Index for all patients in the Royal Melbourne Hospital cohort.
Overall survival according to Disease Risk Index for all patients in the Royal Melbourne Hospital cohort.
We find the DRI to be a simple, practical and robust tool for pre-transplant risk stratification, and estimation of survival and relapse in alloHSCT recipients, when calibrated with local alloHSCT outcome data. However, users should be familiar with its limitations when applied to smaller cohorts or cohorts with shorter follow-up.
References
1. Armand P, Gibson CJ, Cutler C et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 2012;120:905-913.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal