Abstract
There is a growing awareness of the molecular heterogeneity of DLBCL beyond the GC and non-GC well established subtypes. “Double-hit” lymphoma (DHL) harboring rearrangements of c-Myc and BCL2 have been clearly associated with a poor prognosis. It is well recognized that these pts do poorly with R-CHOP and typically cannot be salvaged using ASCT in the relapse setting. Small series suggest that dose-intensive (DI) strategies may lead to better outcomes in the frontline setting and that some pts achieve durable disease control after allogeneic SCT in the relapse setting. We report here one of the largest series of consecutive DHL pts treated with a DI approach followed by allogeneic SCT and compare outcomes to a similar pt population treated with standard dose (SD) chemotherapy.
We conducted a retrospective cohort study of DHL pts to evaluate clinical outcomes stratified by chemotherapy intensity ± SCT treated at John Theurer Cancer Center (JTCC) between 4/09-6/13. DHL was defined by the presence of c-Myc rearrangement (FISH) in combination with rearrangement of BCL2 (FISH) or BCL2 over expression (IHC). Primary study endpoints were PFS and OS assessed by chart review and SSDI database. Investigators were blinded to clinical outcomes. A group of hematopathologists reviewed all cases to verify DHL status (also blinded to outcome). Statistical tests included a two-sample t-test to compare baseline characteristics between groups and Kaplan-Meier and Cox regression survival analyses (med follow up 7.9 months, max follow up 46 months).
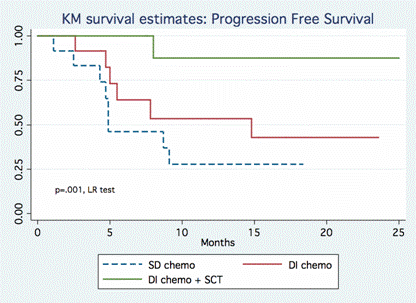
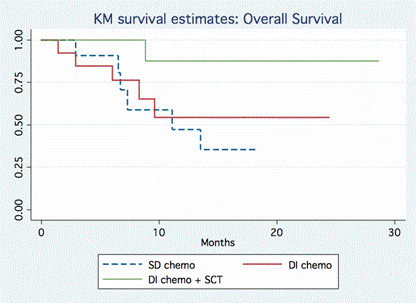
Among the 280 pts with either DLBCL or FL treated between 4/09-6/13 at JTCC, 37 pts (12%) were identified as DHL [100% c-Myc + rearrangement by FISH; 86% t(14;18) + rearrangement by FISH and 95% BCL2 over expression by IHC]. These included 68% de novo DHL: 68% DLBCL, 24% FL, 8% transformed FL and 82% were of germinal center cell origin (Hans). Baseline pt characteristics included med age 60 yr (range 23-84), 57% males, 60% aaIPI ≥3, 93% stage III-IV, 29% BM involvement, 76% bulky disease, 61% extra-nodal disease, med LDH 899 (range 419-8276) and med Ki-67 was 85% (range 20-99%). Treatment regimens included a DI regimen in 66% (n=24) [R-hyper-CVAD, R-CODOX-M/IVAC (Magrath Regimen), DA-R-EPOCH] or a SD regimen in 33% (n=12) [mostly R-CHOP, R-EPOCH (not DA), R-ESHAP]. Of the DI group, 42% of pts underwent SCT as consolidation (73% allogeneic, 27% autologous). Allogeneic SCT included: 25% URD, 75% myeloablative conditioning (Flu/Mel, Cy/TBI, BEAM). There were no significant differences between DI vs. SD groups in terms of pt age, stage, LDH, cell of origin, number of cycles of chemotherapy or Ki-67 (p=NS). Med PFS for the entire cohort was 30.2 months. When stratified by chemotherapy intensity, there was a significant difference in PFS for the DI vs. SD treatment group [46 vs. 8 months, HR 0.26, p=0.005]. The added benefit of SCT was demonstrated when pts were stratified by transplantation status (Figure 1): med PFS for DI with SCT, DI without SCT, and SD were 46, 14.8 and 4.9 months, respectively [DI with SCT vs. SD, HR 0.079, p=0.016; DI without HSCT vs. SD, HR 0.53, p=0.237]. Med OS for SD group was 11.1 months, while med OS for the DI ± SCT groups has not been reached (Figure 2) [DI with SCT vs. SD, HR 0.13, p=0.05; DI without HSCT vs. SD, HR 0.73, p=0.6].
As previously recognized, DHL do very poorly with SD alone. DI strategies with allogeneic SCT lead to significantly longer PFS and OS. Though our series is a retrospective analysis and numbers remain small, this is a relatively large series of “true DHL” (with 100% c-Myc rearrangement by FISH and 86% t(14;18) translocation), a situation where no treatment has demonstrated long term benefit. The effect of DLI (a few relapses post-SCT converted to CR after DLI) and the durability of PFS support a GVL effect of allogeneic SCT in DHL. Prospective screening for DHL is warranted in high risk B-cell NHL particularly based on high Ki-67. Additional studies are needed to confirm the benefit of allogeneic SCT consolidation after DI induction therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal