Abstract
The metabolism of lymphocytes activated in vivo remains poorly understood. Previous work has demonstrated that T cells activated during graft-versus-host disease (GVHD) adopt metabolic profiles distinct from other cell types. We hypothesized that a deeper understanding of allogeneic T cell metabolism, followed by exploitation of metabolic differences, might allow selective elimination of pathogenic T cells while preserving normal immune responses following bone marrow transplantation (BMT). We tested this hypothesis by evaluating fatty acid (FA) metabolism in proliferating donor T cells during a parent into F1 model of GVHD (C57Bl/6 into B6D2F1). Compared to naive donor T cells, T cells from allogeneic recipients increased FA transport (44.3 ±6.9% vs. 0.7 ±0.1%, p=0.003) and up-regulated a regulator of oxidative metabolism, PGC-1α (0.9 ±0.2 vs. 0.03 ±0.01, p=0.005). These changes were present in T cells recovered from both the liver and the spleen. Allogeneic T cells also up-regulated mRNA and protein levels for FA oxidation enzymes (e.g. CPT1a) and oxidized more fatty acids ex vivo. We confirmed these changes in a second, minor histocompatibility model of GVHD, where we observed significantly increased levels of FA transport, PGC-1α, and FA oxidation enzymes.
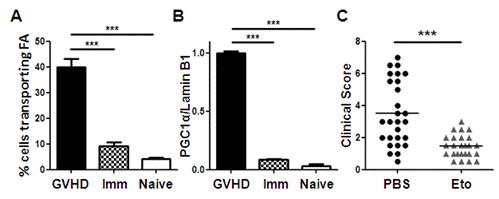
To identify potential therapeutic targets selective for alloreactive T cells, we compared the metabolic profile of allogeneic T cells to the profile observed in T cells following acute activation in vivo. We injected OT-I T cells into naïve C57Bl/6 mice, challenged mice one day later with ovalbumin-bearing dendritic cells, and harvested T cells 7 days after immunization. OT-I T cells proliferated robustly to cellular immunization, but did not increase FA transport or levels of PGC-1α (Figure 1A,B). We then tested the ability of inhibitors of FA oxidation (targeting CPT1a) to selectively eliminate allogeneic T cells during GVHD. A single dose of etomoxir decreased the total number of donor T cells in allogeneic recipients by 35% and eight doses over 14 days significantly improved clinical GVHD scores (Figure 1C). Treatment with a second CPT1a inhibitor improved post-transplant survival (78% vs. 50%, inhibitor vs. PBS respectively). Importantly, CPT1a inhibition did not diminish the number of regulatory T cells or the number of T cells reconstituting via homeostatic proliferation following syngeneic BMT. In total, these data demonstrate that allogeneic T cells increase FA metabolism during GVHD and that this phenotype differentiates allogeneic cells from T cells responding to acute activation or proliferating during homeostatic reconstitution. This study challenges the paradigm of effector lymphocyte metabolism in vivo and we conclude that inhibition of FA oxidation may be a selective way to eliminate pathogenic T cells causing immune-mediated disease.
Changes in fatty acid metabolism allow for selective elimination of pathogenic T cells. T cells were recovered from allogeneic recipients (GVHD), recipients immunized with antigen-bearing dendritic cells (Imm), or naïve donors and assessed for FA transport (A) or levels of the transcriptional co-activator PGC-1α (B). C, Allogeneic recipients were treated for two weeks with the FA oxidation inhibitor etomoxir (Eto), or PBS as a control, and clinical scores assessed for individual mice on days 28, 35, 42, and 49 post-transplant. The average clinical score is represented by a solid line. (*** p<0.0001)
Changes in fatty acid metabolism allow for selective elimination of pathogenic T cells. T cells were recovered from allogeneic recipients (GVHD), recipients immunized with antigen-bearing dendritic cells (Imm), or naïve donors and assessed for FA transport (A) or levels of the transcriptional co-activator PGC-1α (B). C, Allogeneic recipients were treated for two weeks with the FA oxidation inhibitor etomoxir (Eto), or PBS as a control, and clinical scores assessed for individual mice on days 28, 35, 42, and 49 post-transplant. The average clinical score is represented by a solid line. (*** p<0.0001)
Ferrara:University of Michigan: Patent for GVHD Biomarkers, Patent for GVHD Biomarkers Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal