Abstract
The Swedish Myeloma Registry (SMR) is a prospective observational registry designed to document real-world management and outcomes in newly diagnosed myeloma, with the purpose to improve the quality of the management of patients in Sweden. Population-based registries may provide complementary information on the management of patients to that of clinical intervention trials. With high representation and excellent data quality we can present valuable information in a whole population and reduce the impact of selection on outcome and reduce the subsequent problem with extrapolating data from clinical intervention studies on non-study populations.
The registry comprises web-reported data on all patients diagnosed with myeloma, plasmocytoma, and plasma cell leukemia from 2008 in Sweden, at time of diagnosis and after one year of follow-up. Coverage is analyzed through the compulsory Swedish Cancer Registry. Survival is achieved from the Swedish Tax Agency. Missing data are actively requested.
This first report contains data on patients diagnosed between 2008 and 2011 with follow-up after one year on patients with symptomatic disease 2008-2010, with a follow-up through the end of 2012. Analyses of incidence, patient characteristics at baseline, proportion of patients given intensive treatment, obtaining very good partial remission (VGPR) and overall survival (OS) were estimated.
Clinical data at baseline was available for 2494 patients (96% coverage)and 1- year follow-up data on 1427 patients (90% of all symptomatic cases initially reported), from 70 different centers in Sweden. The age adjusted incidence was 6.5 myeloma cases per 100 000 inhabitants and year. The median age was 70 years for men, and 73 years for women (34% younger than 66 years). At diagnosis, 76% were reported as symptomatic myeloma, 18% as smouldering myeloma, 5% plasmocytoma and 1% plasma cell leukemia. IgG-myeloma was most common (59%), followed by IgA (21%), Bence-Jones (13%), non-secretory (4%), IgD and IgM both less than 1%.
Among symptomatic myeloma (n=1910), 76% had osteolytic lesions or compression fractures at diagnosis. Anemia (defined as hemoglobin levels below 10 g/dl) was seen in 33%, impaired kidney function (s-creatinin levels above 173 mmol/l) in 18%, and hypercalcemia in 21% at the time of diagnosis. In patients were ISS was available, 23%, 45% and 32% were in stage I, II, and III, respectively. Previous MGUS was known in 13 % of patients.
Overall, 81 % of patients 65 years or younger received autologous stem cell transplantation (ASCT) and 4% of the elderly population. In the patients aged 65 years and younger, 63% of patients received one of the newer drugs in the first year of treatment, for the patients 66 to 80 years the number was 56%, and 25% of patients above 80 years.
Throughout the study period, an increase in VGPR-rate on initial treatment was observed, more pronounced in younger patients (<66 years), from 35% in 2008 to 46% in 2010. For patients >65 years, the VGPR-rate increased from 17 to 27%.
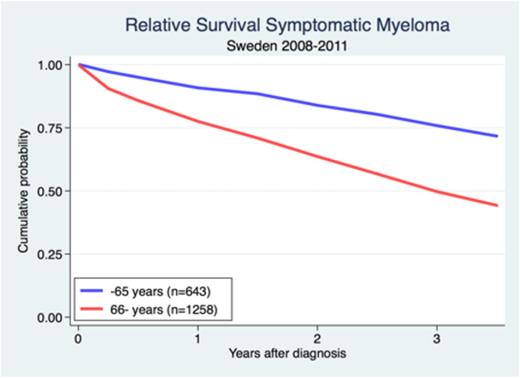
After a median of follow-up time of three years, OS was 63%. There was a significant difference in absolute and relative survival between younger and older patients. In symptomatic myeloma, patients 65 years or younger had an expected 3-year survival of 76% and in patients 66 years and above it was 50% (Figure). The relative 3-year survival for patients with asymptomatic patients was 81%.
Relative survival in symptomatic myeloma patients under and above the age 65 diagnosed in Sweden 2008-2011.
Relative survival in symptomatic myeloma patients under and above the age 65 diagnosed in Sweden 2008-2011.
SMR is an instrument for increased quality in the management of plasma cell neoplasms in Sweden. This first report from the registry shows very high coverage and good adherence to guidelines in all regions of Sweden, both in diagnostics and treatment. A great effort is made to make the SMR complete and to present population-based data on management and outcome in Sweden. Longer follow-up is needed to address the question of the impact of new treatment options on the survival. The registry gives a great opportunity to perform population-based research of high quality based on the acceptance of the registry among treating physicians.
Turesson:Celgene Corp: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal