Abstract
The phase 3 VISTA study demonstrated the superiority of VMP versus melphalan-prednisone (MP) in transplant-ineligible patients with previously untreated MM in terms of response rates, time to progression, and OS (San Miguel et al. N Engl J Med 2008, J Clin Oncol 2013). The protocol-specified duration of VMP therapy was up to 54 weeks (nine 6-week cycles), corresponding to the standard duration of MP therapy. However, it is not known whether this full duration of VMP therapy at the planned bortezomib dose is required for optimal outcomes, and there is no widely accepted clinical guidance on the ideal duration of bortezomib treatment. The aim of this analysis, using data from the VMP arm of the VISTA study, was to determine whether increased cumulative bortezomib dose (total dose received during the study) led to better long-term outcomes.
The VMP regimen in VISTA comprised nine 6-week cycles of bortezomib 1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32 of cycles 1–4 (induction, twice-weekly dosing) and on days 1, 8, 22, and 29 of cycles 5–9 (maintenance schedule, weekly dosing), plus melphalan 9 mg/m2 and prednisone 60 mg/m2 on days 1–4 of all cycles. Dosing of melphalan and prednisone was the same in the MP arm. The maximum planned dose of bortezomib was 67.6 mg/m2, including 41.6 mg/m2 during induction and 26 mg/m2 during the maintenance schedule. A total of 340 patients received at least one dose of bortezomib. The median cumulative bortezomib dose received was 39 mg/m2, which approximately equates to the planned dose during the induction phase of treatment; this was thus selected as the cut-off point for defining patient groups by cumulative bortezomib dose for analyses of OS.
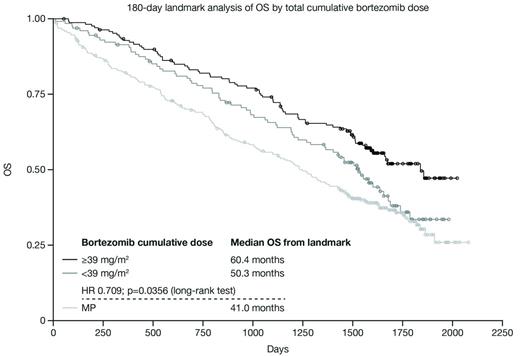
Among all 340 patients, cumulative bortezomib dose ranged from 1.3 to 71.2 mg/m2, and treatment duration ranged from 4 to 424 days. Patient characteristics were well balanced between both high and low cumulative dose groups (Table), although patients in the lower cumulative dose group were older (mean age; t-test, p<0.0001). OS was significantly longer in patients in the higher (≥39 mg/m2) versus lower (<39 mg/m2) cumulative dose group (median 66.3 vs 46.2 months, hazard ratio [HR] adjusted for age 0.561; log-rank test, p=0.0002). There were substantial differences between groups in the reasons for going off treatment; the group of patients who received lower cumulative bortezomib doses displayed a higher incidence of early treatment discontinuations due to adverse events, patient choice, progressive disease, or deaths as compared to patients in the higher cumulative dose group (Table). To overcome the confounding effects of early deaths due to toxicity or other reasons, a landmark analysis of OS was conducted at 180 days among patients alive at that time point according to whether they received a total cumulative bortezomib dose of <39 or ≥39 mg/m2. In this analysis, OS from the landmark remained significantly longer in patients who received a cumulative bortezomib dose of ≥39 vs <39 mg/m2 (median 60.4 vs 50.3 months, HR 0.709, p=0.0356; Figure).
These data indicate that a higher cumulative bortezomib dose, reflecting prolonged treatment duration and/or dose intensity, is associated with improved OS. Maintaining patients on bortezomib therapy, using dose/schedule modifications and adverse event management as required, is thus important in order to maximize cumulative dose and to provide better OS. Early discontinuation was primarily associated with toxicity, and appeared more common in older patients. A less intensive VMP regimen, with limited twice-weekly dosing followed by a less dose-intense bortezomib schedule, could be used to achieve a similar cumulative bortezomib dose and thus maximize treatment duration and outcomes, as shown in Spanish (Mateos et al. Lancet Oncol 2010) and Italian (Palumbo et al. J Clin Oncol 2010) cooperative group trials of alternative VMP regimens.
| . | Cumulative bortezomib dose . | |
|---|---|---|
| Characteristic . | <39 mg/m2 (N=170) . | ≥39 mg/m2 (N=170) . |
| Mean age, years | 74 | 71 |
| Male, % | 50 | 49 |
| White / Asian, % | 86 / 12 | 91 / 8 |
| KPS 60–70 / 80–90 / 100, % | 34 / 52 / 14 | 38 / 48 / 15 |
| β2M <2.5 / 2.5–5.5 / >5.5 mg/L, % | 10 / 58 / 32 | 15 / 52 / 34 |
| Albumin ≥3.5 g/dL, % | 41 | 42 |
| ISS stage I / II / III, % | 18 / 50 / 32 | 19 / 44 / 36 |
| Reasons off treatment, % | ||
| Completed treatment | 31 | 86 |
| Adverse event | 27 | 4 |
| Disease progression | 9 | 5 |
| Patient choice | 17 | 2 |
| Death | 8 | 1 |
| Other | 8 | 4 |
| . | Cumulative bortezomib dose . | |
|---|---|---|
| Characteristic . | <39 mg/m2 (N=170) . | ≥39 mg/m2 (N=170) . |
| Mean age, years | 74 | 71 |
| Male, % | 50 | 49 |
| White / Asian, % | 86 / 12 | 91 / 8 |
| KPS 60–70 / 80–90 / 100, % | 34 / 52 / 14 | 38 / 48 / 15 |
| β2M <2.5 / 2.5–5.5 / >5.5 mg/L, % | 10 / 58 / 32 | 15 / 52 / 34 |
| Albumin ≥3.5 g/dL, % | 41 | 42 |
| ISS stage I / II / III, % | 18 / 50 / 32 | 19 / 44 / 36 |
| Reasons off treatment, % | ||
| Completed treatment | 31 | 86 |
| Adverse event | 27 | 4 |
| Disease progression | 9 | 5 |
| Patient choice | 17 | 2 |
| Death | 8 | 1 |
| Other | 8 | 4 |
β2M = β2-microglobulin; ISS = International Staging System; KPS = Karnofsky performance status
Mateos: Millennium: The Takeda Oncology Company: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees. Richardson:Celgene: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Millennium: The Takeda Oncology Company: Consultancy, Membership on an entity’s Board of Directors or advisory committees. Shi:Millennium: The Takeda Oncology Company: Employment. Niculescu:Millennium: The Takeda Oncology Company: Employment. Elliott:Millennium: The Takeda Oncology Company: Employment. Dow:Millennium: The Takeda Oncology Company: Employment. van de Velde:Janssen Research & Development: Employment; Johnson & Johnson: Equity Ownership. San Miguel:Novartis: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Onyx Pharmaceuticals: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Millennium: The Takeda Oncology Company: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal