Abstract
In the US and EU, B is approved for the treatment of MM using either SC or IV administration. The MMY-3021 study in relapsed/refractory MM pts demonstrated the non-inferiority of SC vs IV B (in terms of response rate after 4 cycles of treatment) and some improvements in the safety profile of B with SC vs IV administration, including lower rates of peripheral neuropathy (Moreau et al, Lancet Oncol 2011). Therefore, SC administration may improve the tolerability of B, potentially impacting patterns of B treatment. The aim of this non-interventional study was to analyze the impact of initial route of B administration on time to dose reduction and dose intensity delivered to pts.
In this retrospective observational cohort study, previously untreated MM pts aged ≥18 yrs who initiated B-based treatment within the McKesson Specialty Health/US Oncology Network between January 1, 2011 and November 30, 2012 and had ≥2 MM-related visits and ≥6 months of follow-up through May 31, 2013 were included. Pts enrolled in randomized clinical trials and/or diagnosed with other cancer were excluded. Data were collected via programmatic data queries of the iKnowMed (iKM) electronic health records (EHR) database. In the base case analysis, the intent-to-treat (ITT) approach was used; baseline pt demographics and disease characteristics, and B dosing patterns (including dose, dose intensity [normalized to dose intensity per month due to asymmetric follow-up periods between groups], rate of dose reduction in the first 16 weeks of treatment, and time to dose reduction) were compared between pts initially receiving SC vs IV B. Some pts switched route of B administration, and sensitivity analyses were conducted using only pts who did not switch. Non-parametric tests were used to test variables described here.
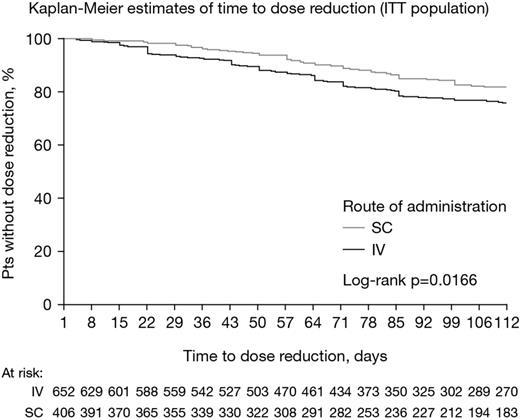
1058 MM pts were included, of whom 652 (62%) initially received IV B, and 406 (38%) initially received SC. Baseline characteristics were generally similar between the IV and SC groups: mean (SD) age was 66 (12) yrs and 67 (12) yrs, with 56% and 60% of pts aged ≥65 yrs, 56% and 54% were male, mean (SD) body mass index was 28.6 (7.0) and 28.1 (5.8), and 18%/27%/44% and 22%/25%/42% had ISS stage I/II/III disease, respectively. Karnofsky performance status (KPS) was poorer in the IV vs SC group, with 8%/30%/26%/27% vs 15%/31%/26%/25% having a KPS of 100/90/80/≤70 (p=0.0164), and the rate of general comorbidities (weight loss, anorexia, dehydration, diarrhea, dyspnea, hyper-/hypovolemia, malaise, nausea, pain, sepsis) at baseline was higher in the IV vs SC group (21% vs 15%, p=0.0178). The groups were well balanced in terms of other comorbidities, practice region, payer status (Medicare, private, other), and MM subtypes. Treatment regimens differed between the IV and SC groups (p<0.0001), and included B+dexamethasone (62% vs 49%) plus lenalidomide (24% vs 28%) or cyclophosphamide (7% vs 17%), B-melphalan-prednisone (4% vs 4%), or other combinations (2% vs 2%). Median dose intensity with IV and SC B was 5.78 mg/m2/month (range 1.05–10.19) and 6.09 mg/m2/month (1.26–11.03), respectively (p=0.02). Overall, 20% vs 14% of pts receiving IV vs SC B had dose reductions in the first 16 weeks (p=0.017). Kaplan-Meier distributions of time to dose reduction are shown in the figure. Among pts with dose reductions, median time to dose reduction with IV vs SC B was 50 days (95% CI: 41, 59) vs 58 days (95% CI: 37, 63), p=0.0332. In total, 243 (23%) pts switched route of administration during treatment – 216 (89%) from IV to SC and 27 (11%) from SC to IV. In analyses excluding these pts, B dose intensity remained lower with IV vs SC (median 5.88 mg/m2/month [range 1.05–10.19] and 6.18 mg/m2/month [1.26–11.03], p=0.0456), rate of dose reductions remained higher (18% vs 13%, p=0.0514), and time to dose reduction remained shorter (median 49 vs 58 days, p=0.0163).
These data indicate that initiation of therapy with SC B is associated with a higher dose intensity per month, fewer dose reductions within 16 weeks, and longer time to dose reduction compared with IV B in the front-line MM setting. When pts who switched route of B administration were excluded from the analyses, similar results were found. As these data continue to mature, further analyses will be required to evaluate in more depth the corresponding clinical outcomes by route of B administration.
Rifkin:Celgene: Membership on an entity’s Board of Directors or advisory committees; Millennium: The Takeda Oncology Company: Membership on an entity’s Board of Directors or advisory committees; Onyx: Membership on an entity’s Board of Directors or advisory committees. Chen:McKesson Specialty Health: Employment. Dhanda:McKesson Specialty Health: Employment. Rembert:McKesson Specialty Health: Employment. Ba-Mancini:Millennium: The Takeda Oncology Company: Employment. Ma:Millennium: The Takeda Oncology Company: Employment. Zhu:Millennium: The Takeda Oncology Company: Employment. Dow:Millennium: The Takeda Oncology Company: Employment. Niculescu:Millennium: The Takeda Oncology Company: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal