Abstract
Prior studies have shown that presence of increased circulating plasma cell (PCs) identified using a slide-based immunofluorescence method is an adverse prognostic marker for overall survival in multiple myeloma (MM), and increases the risk of progression in patients with MGUS and smoldering MM. However, utility in clinical settings has been limited by the cumbersome nature of the test and lack of widespread availability. We studied the prognostic value of circulating PCs using sensitive multiparametric flow cytometry that enable us to quantitatively assess circulating PCs in patients with MM.
We evaluated all newly diagnosed MM patients seen at the Mayo Clinic, Rochester from 2009 to 2011 who had their peripheral blood samples evaluated by flow cytometry prior to therapy. Patients with plasma cell leukemia were excluded. Each blood sample had its peripheral blood mononuclear cells isolated by ficoll gradient and stained with antibodies to CD45, CD19, CD38, CD138 and cytoplasmic Kappa and Lambda Ig light chains. A six-color multi-parameter flow cytometer (Becton Dickinson FacsCantos II) was used to examine each sample with a target of detecting 150,000 events (cells) that was then analyzed using the Facs Diva Software. PCs were selectively analyzed through combinatorial gating using light scatter properties and CD38, CD138, CD19, and CD45. Normal PC's were then separated from clonal PCs based on the differential expression of CD45, CD19 and polytypic Ig light chains. The clonal PCs detected were reported out as the number of clonal events/150,000 collected total events. For those samples where less than 150,000 events were gated or examined, the number of final clonal events was adjusted to 150,000 events. Survival analysis was performed by the Kaplan-Meier method and differences assessed using the log rank test.
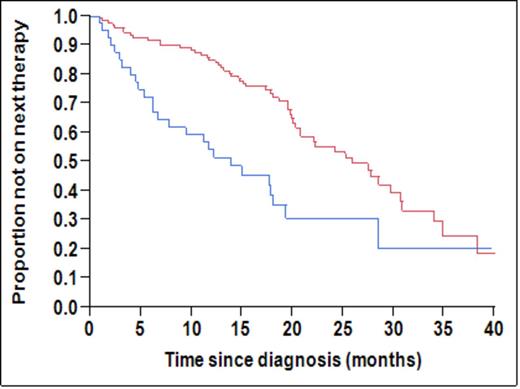
There were 158 consecutive patients with newly diagnosed MM who had their peripheral blood evaluated via flow cytometry as part of their routine clinical evaluation. The median age of this group was 66 years (39-95) and 59% were male. At the time of this analysis, 25 patients had died and the 2-year OS rate for the cohort was 83%. The 2-year OS for the 89 (55%) patients with any circulating PCs was 76% compared with 91% for those with none (P=0.02). The median number of circulating clonal PCs was 33 (range, 0-46,413)/ 150,000 gated events. Using a ROC analysis the best cutoff predicting 1 and 2-year mortality were 435 and 376 events, respectively. Based on this, we defined >=400 events as a cutoff for defining patients with high-risk disease. The median time-to-next-treatment (TTNT) for patients with circulating clonal PCs >=400 (n=37, 23%) was 14 months compared with 26 months for those with <400 events (n=121, 77%) (p<0.001; Fig 1a). The median OS for those with >=400 clonal PC events was 32 months compared with not reached for those with <400 events (p<0.001; Fig 1b). Patients with >= 400 circulating PCs had higher ISS stage, creatinine, LDH, PC labeling index and bone marrow PC% compared with the rest. In a univariate analysis examining the presence of circulating PC >=400, LDH, labeling index, marrow PC% and FISH risk status, only circulating PCs were prognostic for overall survival (P = 0.0011). Among the 89 patients with any circulating PCs, 26 (30%) had CD45+ clonal PCs. The TTNT in patients with a clonal CD45+ population was inferior to those with no CD45+ clonal PCs in circulation (median 11 vs. 19 months, P = 0.034) as was the OS (P = 0.039).
Quantitative estimation of circulating clonal PCs in patients with newly diagnosed MM is a powerful predictor of early relapse from therapy and mortality. A cutoff of >=400 clonal events/150,000 gated mononuclear events predicts for a median TTNT of 14 months and OS of 32 months. This parameter is more powerful than the current high-risk parameters such as FISH risk status as well as traditional high-risk markers such as ISS stage and LDH levels, and is able to identify a group of patients with particularly poor outcome.
Kumar:Celgene: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Onyx: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal