Abstract
Autologous transplant has been shown to prolong survival in patients with MCL, a disease which is known to have poor long-term survival. The purpose of this clinical trial is to reduce the relapse rate and prolong survival through the induction of anti-tumor T cells which can effectively survey, expand and eradicate tumor cells when and if they arise. In a murine model, T cells from CpG-tumor vaccinated donors were shown to expand preferentially in recipient mice following a myeloablative regimen and were effective in eradicating tumor in these mice (Goldstein, M. et al., Blood 2011). Consequently, we developed a clinical trial where patients with mantle cell lymphoma (MCL) are given a CpG-based cellular vaccine followed by an autologous stem cell transplant (Immunotransplant). T cells, harvested after vaccination prior to transplant, are reinfused post-transplant. Blood samples are taken prior to vaccine, post the initial vaccination, as well as post immunotransplant to study the CD4 and CD8 anti-tumor T cell responses. Blood samples are also drawn pre and 2 weeks post a vaccine boost given approximately 6 months post-transplant to assess the memory component of the anti-tumor T cells.
Peripheral blood lymphocytes (PBL) were cultured for 5 days with and without autologous CpG activated tumor. T cells were re-stimulated over-night with fresh activated tumor then analyzed by flow cytometry for phenotype (CD4+ T cells, CD8+/CD56- T cells and memory marker, CD45RO) as well as functionality (cytokine expression [IFN-g, TNF and IL-2], cytolitic activity [perforin and granzyme B expression] and activation markers, CD137 and ICOS [CD278]).
In several cases, tumor-responsive T cells from co-cultures were sequenced to determine their TCRb repertoire. Clonotypes enriched over those found in PBL drawn pre-vaccine were deemed to be antigen-specific. These clones were compared to ones enriched directly in the blood after the original vaccine and later boost.
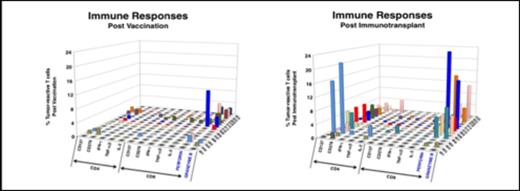
To date, 21 MCL patients have been enrolled and have received the immunotransplant as well as final vaccine boost. 15 patients have been analyzed and have shown anti-tumor T cell responses following vaccine and immunotransplant. In most cases, the T cell responses were boosted post immunotransplant suggesting an expansion of anti-tumor T cells as predicted by the murine model. The phenotype of the responding T cells (CD4, CD8, or both CD4 and CD8) varied between patients. (Figure 1.)
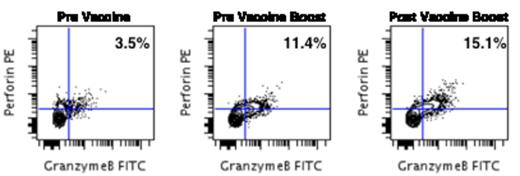
Also, in 11/15 patients, tumor-responsive T cells were still present at the time of the final vaccine, about 6 months post-transplant. The final vaccine boosted T cell responses, mostly in the CD8 compartment, suggesting an induced memory anti-tumor T cell response, which is durable over time. (Figure 2.)
Finally, TCRb clonotypic analyses of the responding T cells demonstrated that these T cell clones were enriched in the blood post vaccine providing further proof that they were vaccine-induced.
Immunotransplant can induce and expand a population of tumor-responsive memory T cells, both CD4 and CD8, which can be detected as an expansion of specific TCRb clonotypes in whole blood. This response is durable over time and can be boosted suggesting a means by which the immune system can be enticed to maintain anti-tumor surveillance and eradicate tumor cells in the future.
Faham:Sequenta, Inc.: Employment, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal