Abstract
MEDI-551 is an affinity-optimized and afucosylated humanized IgG kappa monoclonal antibody directed against CD19 and induces malignant clone destruction by antibody-dependent cellular cytotoxicity. This study evaluates the safety profile and clinical activity of MEDI-551 in patients with relapsed/refractory B-cell malignancies. These include chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and multiple myeloma (MM).
Determine the safety profile and maximum tolerated dose (MTD) of MEDI-551 in patients with relapsed/refractory B-cell malignancies. Secondary objectives include clinical activity of MEDI-551.
In this phase 1/2 open-label multicenter, global dose-escalation and expansion study, patients with relapsed or refractory CLL, DLBCL, FL, or MM received MEDI-551 (at 0.5, 1, 2, 4, 8, or 12 mg/kg) by intravenous infusion administered over 28-day cycles using standard 3+3 dose escalation. Dose escalation continued to the maximum dose ≤12 mg/kg or until MTD was reached. Therapy continued for 2 cycles beyond complete response (CR), or until unacceptable toxicity or disease progression. Dose-limiting toxicity was defined as a MEDI-551-related adverse event (AE) that prevented completion of a full first cycle of MEDI-551, or as a ≥grade 3 toxicity (excluding hematologic toxicity) that could not be ascribed to another cause.
Of 91 patients who received ≥1 dose of MEDI-551, 25 patients (CLL [3], DLBCL [6], FL [12], MM [4]) were enrolled in the phase 1 escalation portion (Jun 2010–Aug 2011). No MTD was achieved. The phase 2 expansion phase included 66 patients (CLL [23], DLBCL [20], FL [22], MM [1]) as of 14Jul2013. Three patients were re-treated with MEDI-551 upon relapse. Median age of patients treated was 66 years; median lines of prior therapy was 6. The median number of treatment cycles was 5 with a maximum of 28 cycles. There were 14 deaths due to AEs (none were drug-related) and 15 subjects discontinued treatment. One subject each discontinued due to drug-related neutropenia and infusion reaction. Most AEs were grade 1/2 with dose-independent frequency and severity (Table). Of 91 patients, 5 (5.5%) patients had grade 4 TEAEs (2 with drug-related neutropenia) and 9 (9.9%) had grade 5 events, none were drug related. Of 19 patients with 38 serious AEs (SAE), 2 patients had 3 events that were considered drug-related; pneumonia and sepsis in 1 patient and infusion related reaction in the other.
Treatment-emergent adverse events with highest severity by frequency (safety population)
| Adverse Event . | Patients,n (%)(N=91) . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 and 5 . |
|---|---|---|---|---|---|
| Fatigue | 27 (29.7) | 15 (16.5) | 11 (12.1) | 1 (1.1) | - |
| Cough | 24 (26.4) | 17 (18.7) | 7 (7.7) | - | - |
| Infusion reaction | 23 (25.3) | 8 (8.8) | 13 (14.3) | 2 (2.2) | |
| Diarrhea | 18 (19.8) | 14 (15.4) | 3 (3.3) | 1 (1.1) | - |
| Nausea | 18 (19.8) | 16 (17.6) | 2 (2.2) | - | - |
| Dyspnea | 16 (17.6) | 11 (12.1) | 4 (4.4) | - | 1 (1.1)* |
| Headache | 15 (16.5) | 10 (11.0) | 4 (4.4) | 1 (1.1) | - |
| Pyrexia | 15 (16.5) | 10 (11.0) | 4 (4.4) | 1 (1.1) | - |
| Back pain | 13 (14.3) | 7 (7.7) | 4 (4.4) | 2 (2.2) | |
| Vomiting | 12 (13.2) | 11 (12.1) | 1 (1.1) | - | - |
| Neutropenia | 11 (12.1) | - | 6 (6.6) | 5 (5.5) |
| Adverse Event . | Patients,n (%)(N=91) . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 and 5 . |
|---|---|---|---|---|---|
| Fatigue | 27 (29.7) | 15 (16.5) | 11 (12.1) | 1 (1.1) | - |
| Cough | 24 (26.4) | 17 (18.7) | 7 (7.7) | - | - |
| Infusion reaction | 23 (25.3) | 8 (8.8) | 13 (14.3) | 2 (2.2) | |
| Diarrhea | 18 (19.8) | 14 (15.4) | 3 (3.3) | 1 (1.1) | - |
| Nausea | 18 (19.8) | 16 (17.6) | 2 (2.2) | - | - |
| Dyspnea | 16 (17.6) | 11 (12.1) | 4 (4.4) | - | 1 (1.1)* |
| Headache | 15 (16.5) | 10 (11.0) | 4 (4.4) | 1 (1.1) | - |
| Pyrexia | 15 (16.5) | 10 (11.0) | 4 (4.4) | 1 (1.1) | - |
| Back pain | 13 (14.3) | 7 (7.7) | 4 (4.4) | 2 (2.2) | |
| Vomiting | 12 (13.2) | 11 (12.1) | 1 (1.1) | - | - |
| Neutropenia | 11 (12.1) | - | 6 (6.6) | 5 (5.5) |
Grade 5
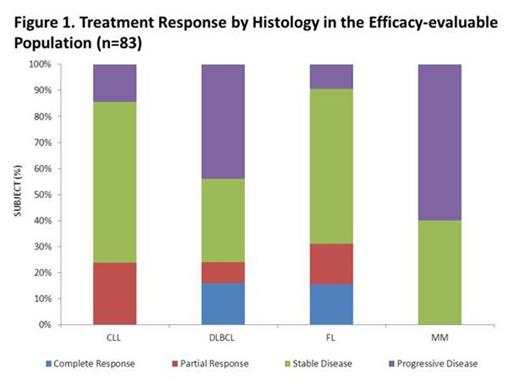
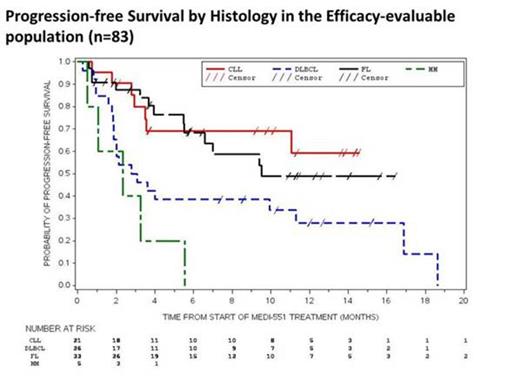
Of 83 patients in the efficacy evaluable population (includes all patients who received any treatment of MEDI-551 and completed at least 1 post-baseline disease assessment), 9 had CR, 12 had partial responses (PR) and 42 had stable disease (SD; Figure 1). ORR to single-agent MEDI-551 was 24%, 24%, or 31% respectively in heavily pre-treated patients with CLL, DLBCL, or FL. Median progression-free survival was ≈9 months (Figure 2).
MEDI-551 has an acceptable safety profile warranting further study. Anti-tumor activity was achieved in a heavily pre-treated population of DLBCL, CLL, and FL patients respectively in this single-agent study. Phase 2 studies of MEDI-551 in combination with chemotherapy in DLBCL and CLL are ongoing.
This study was sponsored by MedImmune.
Forero-Torres:MedImmune: Research Funding. Hamadani:MedImmune: Research Funding. Fanale:MedImmune: Research Funding. Bello:MedImmune: Research Funding. Kipps:MedImmune: Research Funding. Offner:MedImmune: Research Funding. Verhoef:MedImmune: Research Funding. Federico:MedImmune: Research Funding. Gregory:MedImmune: Research Funding. Sonet:MedImmune: Research Funding. Assouline:MedImmune: Research Funding. Pérez de Oteyza:MedImmune: Research Funding. Tomas:MedImmune: Research Funding. Cuneo:MedImmune: Research Funding. Elgeioushi:MedImmune: Employment, Stock/stock options from AstraZeneca Other. Goswami:MedImmune: Employment, Stock/stock options from AstraZeneca Other. Ibrahim:MedImmune: Employment, Stock/stock options from AstraZeneca Other. Herbst:MedImmune: Employment, Stock/stock options from AstraZeneca Other. Cheson:MedImmune: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal