Abstract
The highly aggressive plasmablastic lymphoma (PBL), originally described almost exclusively in HIV+ patients, was nearly uniformly fatal in the pre-HAART era. We hypothesized that aggressive chemotherapy and HAART could result in cures.
We retrospectively analyzed baseline characteristics, treatment patterns and outcomes of patients (pts) with PBL treated at multiple centers within the AIDS Malignancy Consortium (AMC). HIV positivity was not required. 19 confirmed PBLs from 9 national AMC sites diagnosed between 1999 and 2008 were evaluated.
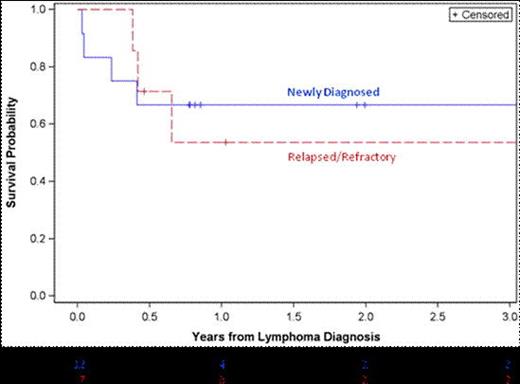
17/19 patients (pts) with confirmed PBL were HIV+. Data was captured at initial diagnosis on 12 pts (all HIV+) and 7 with relapsed/refractory disease (5 HIV+). HAART status at PBL initial diagnosis was 33% on, 58% off, and unknown 8%. Median CD4 count 110 (range 4-658). First line chemotherapy was given to 10/12 (83%) newly diagnosed patients with stage I/II (6) vs III/IV(6) disease. This was CHOP(4), CDE (1), EPOCH (2) and EPOCH with high dose methotrexate and zidovudine (2). Second line therapy was given to 5/7 relapsed/refractory patients with stage I (1) vs Stage III/IV (5) disease and a median CD4 83 (range 10-202): EPOCH alternate HD Mtx+AZT (n=1); Hyper-CVAD (n=2); High dose Mtx + AZT (n=1); VACOP-B(n=1). One pt underwent BEAM based autologous stem cell transplant. For both groups combined, 6 patients experienced grade 3/4 toxicity. Febrile neutropenia was the most common grade 3/4 toxicity (4 patients) followed by thrombocytopenia (3 patients). One patient with refractory disease experienced grade 5 toxicity. For the 12 newly diagnosed patients, 8 patients were alive at last follow-up and 4 had died. Median follow-up for survivors was 73 (range, 40-165) weeks. One-year survival was 66.7% (SE, 13.6). See Figure 1. For the 7 relapsed/refractory patients, 2 patients were alive at 24 and 54 weeks, and 1 was lost to follow-up. One-year survival was 53.6% (SE, 20.1%).
In the HAART era, aggressive treatment of PBL can result in significant survival times.
However, determination of the superior treatment regimen could not be determined from this small patient sample. CTSU 9177 is prospectively studying PBL with EPOCH.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal