Abstract
There is an increasing amount of data showing that both host immunity and tumor microenvironment play an essential role in determining the clinical course in patients with Hodgkin lymphoma (HL) and may also influence their response to therapy. Some investigators have considered the absolute lymphocyte count (ALC) as a surrogate bio-marker of tumor-infiltrating lymphocytes reflecting systemic host immunity, while the absolute monocyte count (AMC) is regarded as a surrogate bio-marker of tumor-associated macrophages reflecting the tumor microenvironment. For many years lymphopenia has been considered as a negative factor predicting poor outcome in HL. Recently monocytosis and the lymphocyte-monocyte ratio (LMR= ALC/AMC), which combines these two surrogate bio-markers, have also been shown to be useful prognostic parameters in HL, particularly in terms of progression free survival (PFS).
The aim of the present study was to verify whether AMC and LMR are indeed valid prognostic parameters in HL, utilizing a large cohort of patients with the disease.
We collected data from 1079 patients with classical Hodgkin's lymphoma (cHL) enrolled in different clinical trials of “Gruppo Italiano Studio Linfomi (GISL)” during 1988-2007. Previously we had evaluated a database of 371 patients with cHL and examined different AMC and LMR cut-off levels using the maximum log rank test and c-Harell (area under the curve). We identified AMC of 600/mm3 and LMR of 3.5, as optimal cut-off values.
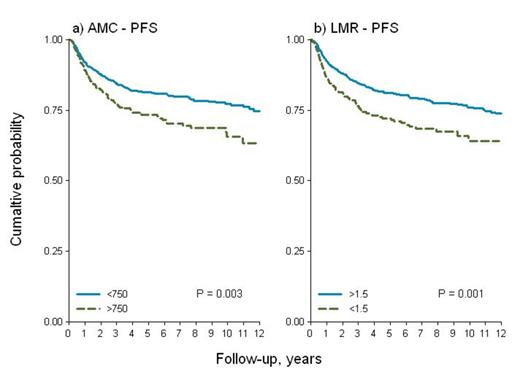
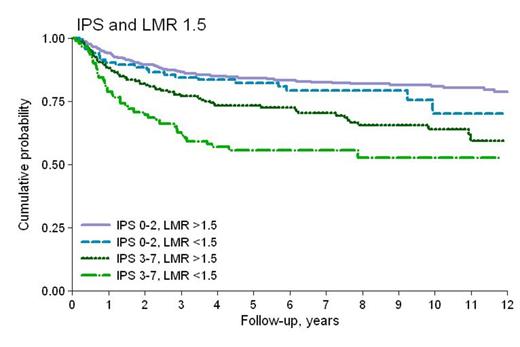
Patients in our current study had the following characteristics: median age 32 years (range 17-71), 51% males, 15% stage IV disease, 74% with International Prognostic Score (IPS) 0-2, 72% nodular sclerosis and 18% mixed cellularity; 43% were treated with ABVD and 29% with BEACOPP/BEACOPP-like regimens. Radiotherapy (RT) was administered to 66% of cases. Median follow-up was 6.8 years (range 0.1-19), and 5-years and 10-years overall survival (OS) was 89 and 83%, respectively; 5-years PFS was 79%. In an earlier evaluation the 10-years PFS for patients with AMC >600/mm3 and LMR <3.5 was significantly worse, compared to the group with AMC <600/mm3 and LMR >3.5 (p-value: 0.032 and 0.005). However now after applying the same cut-off values to the larger database, we found no statistical differences in PFS ,and both threshold values for AMC (600/mm3) and LMR (3.5) lost their prognostic power. Using the same statistical methods for the cohort of 1079 patients, we found different cut-off values for AMC and LMR, that were equally effective in splitting different survival patterns. For AMC the value of 750/mm3 (26% of patients with AMC >750/mm3), and for LMR a ratio of 1.5 (19% of patients with LMR <1.5) revealed a difference in PFS which was statistically significant (p-value 0.003 and 0.001, respectively), as shown in Figure 1. In multivariate analysis after adjusting by IPS, RT and type of chemotherapy regimen administered, AMC and LMR maintained their prognostic significance for PFS (HR 1.37, p 0.022 and 1.37, p 0.037). After interaction with IPS (Figure 2) LMR showed an uniform pattern within the two risk -groups (overall: HR=1.42, p 0.019; IPS 0-2: HR=1.34, p 0.181; IPS 3-7: HR=1.49, p 0.048).
PFS stratified by AMC and LMR cut-offs for 1079 patients.
PFS stratified by AMC and LMR cut-offs for 1079 patients.
PFS stratified by IPS groups and LMR
Our study of 1079 patients confirms that peripheral monocyte and lymphocyte counts have an important prognostic impact in cHL. The cut off-values we used, although different from those utilized previously, were able to distinguish patients with different PFS. The cut-offs reported in the literature vary according to the different authors and the various sets of patients examined. At present we feel there is still a clear need to finally establish definitive and more appropriate cut-off levels that can be used for all future studies. In order to achieve this goal, it is essential to study an even larger series of patients within the framework of an international collaborative study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal