Abstract
Venous thromboembolism (VTE) is a common occurrence in cancer patients. It is a leading cause of death, coming second only to cancer progression (Khorana et al 2007). The risk is further increased by admission to hospital, surgery and administration of chemotherapy (Heit et al 2000, Kröger et al 2006). Thrombotic complications can delay or interfere with cancer therapy, prolong hospitalization and lead to increased use of resources (Carrier et al 2009).
Myeloma, a malignant disorder characterised by the clonal proliferation of plasma cells, has long been linked to VTE (Catovsky et al 1970). VTE in patients with myeloma came to prominence with the advent of immunomodulatory agents such as thalidomide and lenalidomide which dramatically improved outcomes at the expense of complications such as VTE (Musallam et al 2009, Carrier et al 2011).
Prediction and prophylaxis of VTE in the cancer setting has come to the forefront of cancer care with both the American Society of Clinical Oncology and the International Society of Thrombosis and Haemostasis issuing guidelines in 2013 (Lyman et al 2013, Farge et al 2013). Myeloma specific guidelines are also available (Palumbo et al 2008).
The aim of this study was to investigate hematologists' attitudes to VTE and thromboprophylaxis in patients with myeloma by means of a national survey.
The survey was constructed by the investigators and piloted locally to assess reliability. This resulted in a 9 question survey. A list of hematologists working in the Republic of Ireland was obtained from the Irish Haematology Society. Recipients had the option of completing the survey online or by mail.
Six surveys were completed online and 24 by mail. This resulted in a response rate of 67%. Twenty-seven responses met the minimum requirement for inclusion in analysis (90% complete).
The median (IQR) number of patients per respondent was 30 (15-50). All respondents used thromboprophylaxis in at least some of their patients and 26% always used thromboprophylaxis. Forty-eight percent felt that VTE affected <5% of their patients, 22% felt it affected 5-10% and 26% felt it affected 10-20%. The main reasons for commencing patients on thromboprophyaxis were use of Immunomodulatory agents and hospitalisation. Disease burden (ISS, paraprotein level) did not commonly factor into the risk stratification process. Forty-eight percent used published guidelines – the most commonly used was the International Myeloma Working Group guideline.
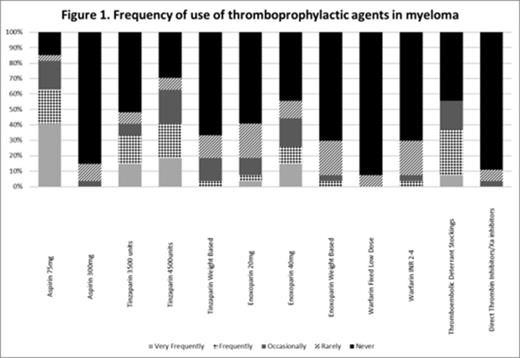
Aspirin 75mg and Tinzaparin 4500 units were the most commonly used regimens – See Figure 1 .
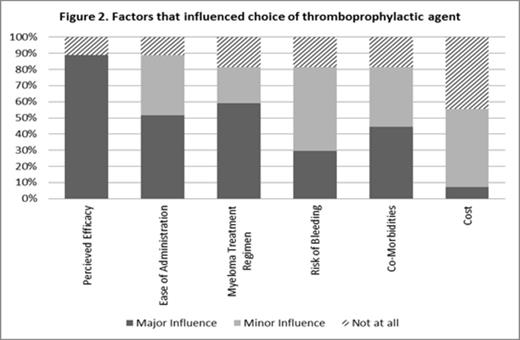
Perceived efficacy was the factor that had the greatest influence on the choice thromboprophylactic agent – See Figure 2 .
Bleeding was not felt to be a significant issue with 67% stating that major bleeding was never an issue and 70% stating that clinically relevant non-major bleeding and minor bleeding were rarely an issue.
To our knowledge, this is the first national survey addressing the issue of thromboprophylaxis in patients with myeloma. While the survey was anonymous, some respondents included identifying details allowing the authors to conclude that a broad cross-section of hematologists responded, limiting the non-response error and making it likely to represent what is occurring in Ireland at present.
It demonstrated variations in practice between hematologists with a wide variety of thromboprophylactic agents being used (Figure 1 ). The lower prophylactic doses of Low Molecular Weight Heparin (Tinzaparin 3500 units/Enoxoparin 20mg) were commonly used despite the higher dose being recommended. Despite not being advocated by guidelines, a small proportion had used fixed low dose warfarin and the new oral anticoagulants.
One institution had formulated their own guideline and all hematologists in that institution claimed to adhere to that guideline highlighting that wide stakeholder involvement increases interest, motivation and support for implementation (McCarlie et al 1999).
Given that more than half of respondents were not using published guidelines to direct thromboprophylaxis in patients with myeloma, there is a role for a national guideline which is based on currently published guidelines with significant stakeholder contributions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal