Abstract
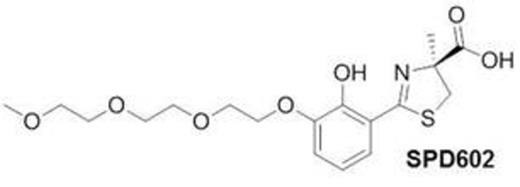
Here, we present a comparison of clinically relevant physicochemical data for deferoxamine, a hexadentate chelator, deferiprone, a bidentate chelator and deferasirox and SPD602, both tridentate chelators, using techniques to allow direct comparison between chelators, which has not previously been possible due to differences in experimental methodology. Broadly speaking, all four compounds possess a high selectivity for iron(III) (Table) and thereby are associated with a strongly negative ligand–iron oxidation potential and thus a low tendency to redox cycle (Ma et al. Curr Med Chem 2012;19:2816). The pFe3+ value (1 µM Fe3+; 10 µM Ligand (L); pH 7.4) for SPD602 is very close to that of deferasirox, larger than that for deferiprone but smaller than found for deferoxamine, suggesting that SPD602 has at least equivalent affinity for the physiologically relevant form iron(III) as the clinical 1st line treatment deferasirox and has a low potential for cell damaging redox cycling activity. The separation in binding affinities between iron(II) and iron(III) for SPD602 was determined to be approximately 14 log units in favour of iron(III). For zinc(II) and copper(II) we have compared the logK1 values corresponding to the two tridentate ligands (Table) as the reported values of the two corresponding logK2 values for deferasirox are unreliable due to polymer formation (Heinz et al. Angew Chem Int Ed 1999;38:2568). Based on the logK1 values, SPD602 showed lower binding affinity for these two metals than deferasirox.
| . | Fe3+ . | pFe3+ . | Mg2+ . | Ca2+ . | Cu2+ . | Zn2+ . |
|---|---|---|---|---|---|---|
| Deferoxamine | 28.3 (logK1) | 26.5 | 4.3 (logK1) | 2.4 (logK1) | 7.9 (logK1) | 5.4 (logK1) |
| Deferasirox | 36.9 (logβ2) | 22.5 | 7.6 (logK1) | 5.5 (logK1) | 18.8 (logK1) | 13.3 (logK1) |
| SPD602 | 33.1 (logβ2) | 22.3 | 6.0 (logK1) | 4.5 (logK1) | 14.8 (logK1) | 9.6 (logK1) |
| Deferiprone | 37.0 (logβ3) | 20.5 | 9.1 (logK1) | 2.0 (logK1) | 19.1 (logβ2) | 13.5 (logβ2) |
| . | Fe3+ . | pFe3+ . | Mg2+ . | Ca2+ . | Cu2+ . | Zn2+ . |
|---|---|---|---|---|---|---|
| Deferoxamine | 28.3 (logK1) | 26.5 | 4.3 (logK1) | 2.4 (logK1) | 7.9 (logK1) | 5.4 (logK1) |
| Deferasirox | 36.9 (logβ2) | 22.5 | 7.6 (logK1) | 5.5 (logK1) | 18.8 (logK1) | 13.3 (logK1) |
| SPD602 | 33.1 (logβ2) | 22.3 | 6.0 (logK1) | 4.5 (logK1) | 14.8 (logK1) | 9.6 (logK1) |
| Deferiprone | 37.0 (logβ3) | 20.5 | 9.1 (logK1) | 2.0 (logK1) | 19.1 (logβ2) | 13.5 (logβ2) |
Table shows iron chelator metal-binding data (stability constants and pFe3+ values) for physiologically relevant metals
In patients with iron overload disorders, levels of iron exceed the iron binding capacity of transferrin, and non-transferrin bound iron (NTBI) is evident. Pre-clinically, SPD602 is efficient at mobilising iron from both mononuclear and oligonuclear iron(III) citrate and albumin-bound iron(III), which are believed to be the major components of NTBI. These data suggest that SPD602 has clinical utility in mobilising the NTBI pool in iron overload.
There are differences in the partition coefficients (logD7.4) of SPD602 (L, -1.3; FeL2, -2.7) and deferasirox (L, 1.1; FeL2, < -3.0) and so whereas deferasirox is hydrophobic as the free ligand, SPD602 is relatively hydrophilic. The clinical implications of this difference in physicochemical properties are unknown. One area where the difference in hydrophobicity is reflected is in the albumin-binding properties: both the free ligand and FeL2 complex of deferasirox are almost completely bound to albumin (> 99%) whereas, in contrast, SPD602 (as the free ligand) is bound less extensively (85.5%).
In summary, SPD602 is an iron chelator with good selectivity for iron(III) over other physiologically relevant metal ions, such as zinc(II) and copper(II), and has identical affinity for iron(III) as the clinical 1st line treatment, deferasirox. In models of NTBI, SPD602 was effective at mobilising iron under physiologically relevant conditions. Further pre-clinical studies are warranted to elucidate additional mechanistic features that may be of clinical importance.
Hider:Shire: Consultancy, Research Funding. Kong:Shire: Research Funding. Luker:Shire: Employment. Conlon:Shire: Employment. Harland:Shire: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal