Abstract
Recent advances in NMA haploBMT have expanded potentially curative treatment options for pts lacking a matched donor. However, there is a paucity of data on the safety and efficacy of haploBMT in pts aged ≥ 60 y. This study evaluated the impact of older age on outcomes of NMA haploBMT with PT/Cy.
We retrospectively analyzed the outcomes of 273 consecutive pts with poor-risk or advanced hematologic malignancies, aged 50-75 y, who received NMA related haploBMT with PT/Cy at Johns Hopkins. Eligibility included ECOG PS ≤ 1 or 2, LVEF ≥ 35%, adequate pulmonary and renal function and no uncontrolled infection. Morphologic CR was required for acute leukemia, ≥ PR for aggressive lymphoma. All pts received Cy (14.5 mg/kg IV days -6 and -5), fludarabine (30 mg/m2 IV days -6 to -2), TBI (200 cGy day -1) and T-cell replete bone marrow (272 pts) or peripheral stem cells (1 pt). GVHD prophylaxis consisted of high-dose PT/Cy (50 mg/kg IV) either once (day 3; 10 pts) or twice (days 3 and 4; 263 pts), mycophenolate mofetil and tacrolimus. Maintenance therapy was permitted and included rituximab in 55/126 B-NHL or CLL cases (44%).
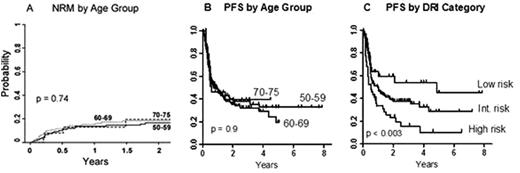
Of the 273 pts (median age 61 y, range 50-75), 154 were aged ≥ 60, including 27 pts aged 70-75. Diagnoses were aggressive lymphoma (79 pts), mantle cell lymphoma (25), indolent lymphoma or CLL (41), acute leukemia or lymphoblastic lymphoma (76), MDS (20), MPD (17), Hodgkin lymphoma (8) or multiple myeloma (7). Forty-one pts (15%) had prior autoBMT, and 138 (51%) had a high-risk comorbidity index score (HCT-CI ≥ 3). There were no statistically significant differences in HCT-CI risk category, histology (lymphoid vs myeloid), graft dose or CMV mismatch according to age by decade. By Disease Risk Index (DRI) category (Blood 2012; 120: 905), 32 pts (12%) were low-risk, 197 (72%) intermediate-risk, 44 (16%) high-risk and none very high-risk. No pts aged ≥ 70 were in the low-risk group. a) Overall results: With a median follow-up of 2.1 (range < 1 - 7.9) y in event-free pts, 2-y probabilities of PFS and OS were 39 (95% CI, 32-44)% and 53 (47-60)%, respectively. The probability of neutrophil recovery was 89% at day 30 (median 16 days). The probability of platelet recovery ≥ 20,000/µL was 85% at day 60 (median 26 days). Graft failure occurred in 12% of evaluable pts, with autologous count recovery in most cases. By competing-risk analysis, the 180-day probability of nonrelapse mortality (NRM) was 11 (95% CI, 7-14)%, grade 2-4 acute GVHD was 32 (27-38)%, and grade 3-4 acute GVHD was 3 (1-6)%. The 1-y probabilities of NRM and chronic GVHD were 14 (95% CI, 10-19)% and 12 (8-16)%. b) Age-specific results: Notably, as compared to age 50-59, we found no statistically significant association between advanced pt age and either NRM (fig A) or PFS. On univariate analysis, older age relative to ages 50-59 was associated with a tendency toward more grade 2-4 acute GVHD, without an evident increase in severe acute GVHD. The 2-y PFS probabilities for pts in their 50's, 60's and 70's were 39%, 36% and 39%, respectively (p = 0.9; fig B), with corresponding 2-year OS probabilities of 51%, 56% and 44 % (p = 0.9). In univariate analyses, no statistically significant association was seen between PFS and older age as a continuous variable (HR 1.01, 95% CI 0.99-1.03, p = 0.44) or categorical variable (ages 60-69 and ≥ 70, relative to 50-59). In contrast, the DRI category was significantly associated with both PFS (p < 0.003; fig C) and OS (p = 0.01). Likewise, in a multivariate analysis adjusted for year of BMT, the DRI was independently associated with PFS (p = 0.047). However, in multivariate analyses of PFS and NRM, no statistically significant association was seen with older age.
These data suggest that in NMA related haploBMT with PT/Cy, advanced pt age is not associated with prohibitive toxicities. In fact, there was no apparent decrement in overall outcome in pts aged ≥ 70 when compared to those in their 50's. Additionally, the DRI is useful for risk stratification. Advanced age is thus no longer a barrier to successful outcomes with partially HLA-mismatched BMT.
Off Label Use: Posttransplantation cyclophosphamide for GVHD prophylaxis.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal